Periodic Classification of Elements
Based on Class 10 -Science & Technology-Chapter 2- Maharashtra Board
Notes
|
Topics to be learn :
|
Types of matter : The types of matter are solid, liquid, gas and plasma.
Types of elements : The types of elements are metals, nonmetals and metalloids.
Atoms : The smallest particles of matter are called atoms.
Difference between the molecules of elements and compounds: 2-An element cannot be decomposed into simple substances by any chemical reaction or simple physical process, e.g. copper, iron, oxygen. 2-The constituents of a compound can be separated by a chemical process, e.g. salt, water and sugar.
Elements :
Compound :
1-Elements contain only one kind of atoms in the free state or combined state.
1-A compound is produced by a chemical reaction of two or more elements.
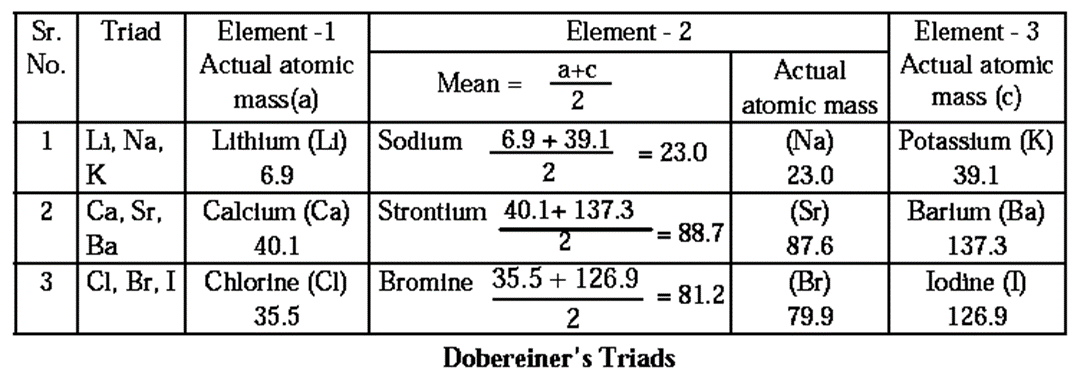
Dobereiner’s Triads : In the year 1817 Dobereiner (a German scientist) proved that the properties of elements are related to their atomic masses.
He made groups of three elements each, showing similar chemical properties and called them triads.
He arranged the three elements in a triad in an increasing order of atomic mass and showed that the atomic mass of the middle element was approximately equal to the mean of the atomic masses of the other two elements.
Newlands’ Law of Octaves : In the year 1866, Newlands arranged the elements known at that time in increasing order of their atomic masses, he found that every eighth element had properties similar to those of the first. For example, sodium is the eighth element from lithium and both have similar properties.
Limitations of Newlands’ Octaves :
Many limitation were found in Newlands’ octaves.
- Newland could arrange elements only up to calcium, out of the total 56 elements known.
- After calcium, every eighth element did not possess properties similar to that of the first.
- Only 56 elements were known at the time of Newlands, but later several elements were discovered.
- In order to fit the existing element arrangement, Newlands placed two elements in the same position which differed in their properties.
- For example Iron, an element which resembles cobalt and nickel in its properties is placed far away from these elements.
- The periodic table did not include inert gases because they were not discovered then.
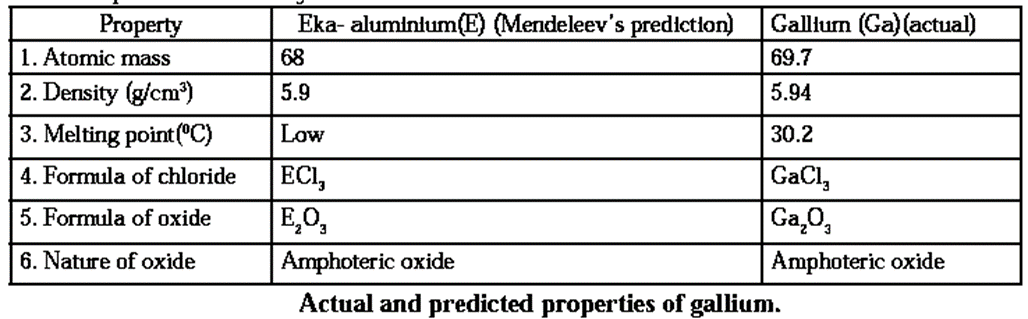
Mendeleev’s Periodic table: The most important step in the classification of elements in Mendeleev’s periodic table is the fundamental property of elements, namely, the atomic mass, as standard.
- He arranged 63 elements known at that time in an increasing order of their atomic masses.
- Then he transformed this into the periodic table of elements according to their physical and chemical properties.
- Mendeleev found that the elements with similar physical and chemical properties repeat after a definite interval.
Mendeleev’s periodic law: The elements with physical and chemical properties are a periodic function of their atomic masses.
The vertical columns in the periodic table are called groups while the horizontal rows are called periods.
Merits of Mendeleev’s periodic table: Demerits of Mendeleev’s periodic table :
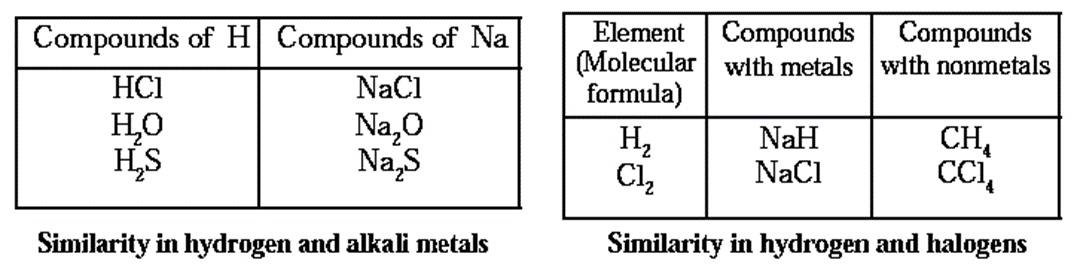
Modern Periodic Law :
After the discovery of electron, scientists started exploring the relation between the electron number of an atom and the atomic number.
The atomic number in Mendeleev’s periodic table only indicated the serial number of the element.
Henry Moseley showed that the atomic number of an element is the most fundamental property and not its atomic mass.
- Accordingly Mendeleev’s Periodic law was modified into Modern Periodic law and it can be stated as : The chemical and physical properties of elements are a periodic function of their atomic numbers.
Structure of the Modern Periodic Table :
Modern Periodic Table and electronic configuration of the elements : The characteristics of the groups and periods in the modern periodic table are because of electronic configuration of the elements.
- It is the electronic configuration of an element which decides the group and the period in which it is to be placed.
- The neighbouring elements within a period differ slightly in their properties while distant elements differ widely in their properties.
- Elements in the same group show similarity and gradation in their properties.
Groups and electronic configuration :
Characteristics of the Groups and Periods : Various properties of all the elements in a group show similarity and gradation. However, the properties of elements change slowly while going from one end to the other (for example, from left to right) in a particular period.
- The number of valence electrons in all these elements from the group 1, i.e. the family of alkali metals, is the same. Similarly, the element from any other group, the number of their valence electrons to be the same. For example, the elements beryllium (B9), magnesium (Mg) and calcium (Ca) belong to the group 2, i.e. the family of alkaline earth metals.
- There are two electrons in their outermost shell the number of valence electrons are 2. Similarly, there are seven electrons in the outermost shell of the elements such as fluorine (F) and chlorine (Cl) from the group 17, i.e. the family of halogens.
- While going from top to bottom within any group, one electronic shell gets added at a time. From this, the electronic configuration of the outermost shell is characteristics of a particular group.
In the modern periodic table :
- Elements are arranged in an increasing order of their atomic numbers.
- Vertical columns are called groups. There are 18 groups. The chemical properties of the elements in the same group show similarity and gradation.
- Horizontal rows are called periods. There are in all 7 periods. The properties of elements change slowly from one end to the other in a period.

Periods and electronic configuration :
- In modern periodic table, there are seven horizontal rows called periods. In a period, the change in valency of an element varies with electronic configuration.
- The number of valence electrons is different in these elements. However, the number of shells is the same. In a period, while going from left to right, the atomic number increases by one at a time and number of valence electrons also increases by one at a time. In a period, there is gradation in properties of elements.
- The elements with the same number of shells occupied by electrons belong to the same period. The elements in the second period, namely, Li, Be, B, C, N, O, F and Ne have electrons in the two shells, K and L. The elements in the third period, namely, Na, Mg, Al, Si, P, S, Cl and Ar have electrons in the three shells; K, L and M.
The chemical reactivity of an element is determined by the number of valence electrons in it and the shell number of the valence shell.
Periodic trends in the modern periodic table : When the properties of elements in a period or a group of the modern periodic table are compared, certain regularity is observed in their variations. It is called the periodic trends in the modern periodic table. The periodic trends in only three properties of elements; namely, valency, atomic size and metallic-nonmetallic character is studied.
Distinguish between Groups and Periods : of electrons in the outermost shell of an atom of an element belonging to that group. of electronic shells present in an atom of an element belonging to that period. show similar properties, but their chemical properties gradually change from left to right in a period.
Groups
Periods
The vertical columns of elements in the modern periodic table are called groups.
The horizontal rows of elements in the modern periodic table are called periods.
The group number indicates the number
The period number indicates the number
The elements in the same group show similar chemical properties.
The elements in the same period do not
Valency: The valency of an element is determined by the number of electrons present in the outermost shell of its atoms, that is, the valence electrons.
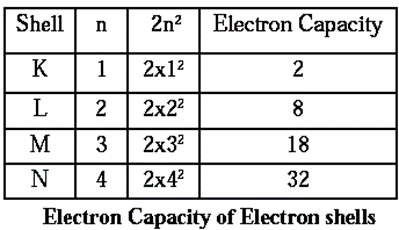
Q. What are the values of n & electron capacity for the shells K, L M and N?
Ans. For the shell K, the value of n is 1 Electron capacity (2x12 ) = 2 For the shell L, the value of n is 2 Electron capacity (2x22 ) = 8. For the shell M, the value of n js 3 Electron capacity (2x32 ) = 18 For the shell N, the value of n js 4 Electron capacity (2x42 ) = 32
Q. What is the maximum number of electrons that can be accommodated in a shell? Write the formula.
Ans. The maximum number of electrons that can be accommodated in a shell is 32 electrons. The formula is 2n2.
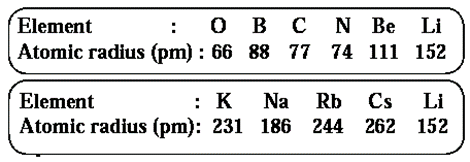
Atomic size : The size of an atom is indicated by its radius. Atomic radius is the distance between the nucleus of the atom and its outermost shell.
- Atomic radius is expressed in the unit picometre (pm) which is smaller than nanometre (1 pm = 10-12 m).
- Atomic size depends on number of shells of an atom. More the number of shells larger is the atomic size.
- While going down a group the atomic size goes on increasing. This is because while going down a group a new shell is added. Therefore the distance between the outermost electrons and the nucleus goes on increasing. As a result of this the atomic size increases.
- While going from left to right within a period atomic radius goes on decreasing and the atomic number increases one by one, that means positive charge on the nucleus increases by one unit at a time.
- However, the additional electron gets added to the same outermost shell. Due to the increased nuclear charge the electrons are pulled towards the nucleus to a greater extent and thereby the size of the atom decreases.
Some elements and their atomic radii are given here:
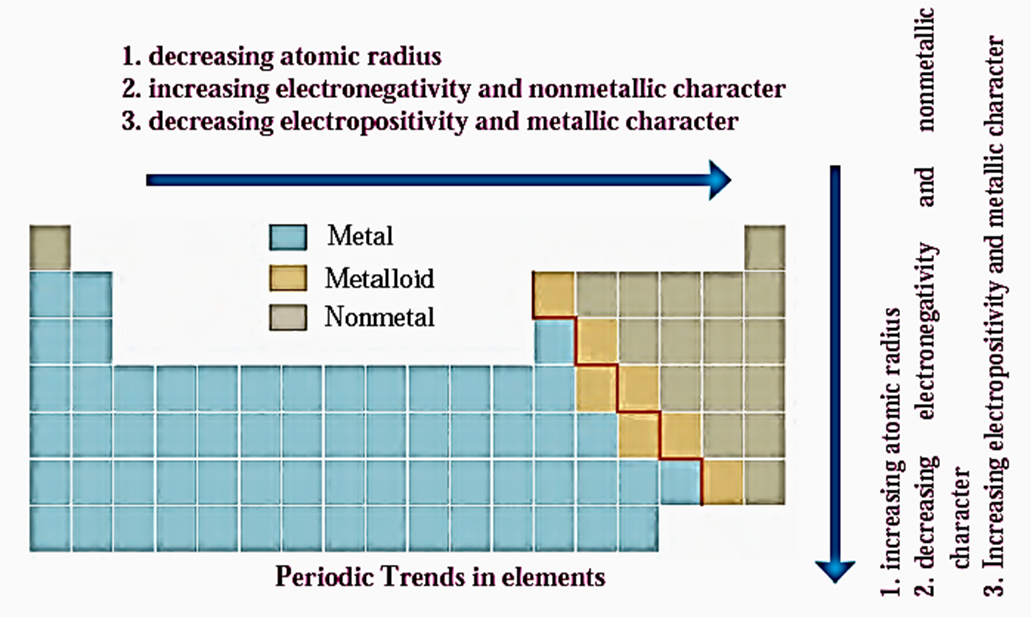
Metallic-Nonmetallic character : It is observed that the metallic elements like sodium, magnesium are towards the left. The nonmetallic elements such as sulphur, chlorine are towards the right.
- The metalloid element silicon lies in between these two types.
- It is observed that the zig-zag line separates the metals from nonmetals in the periodic table.
- Elements are arranged in such a way that metals are on left side of this line, nonmetals on the right side and metalloids are along the border of this line.
- It is observed from the chemical formulae of simple ionic compounds that the cation in them is formed from a metal while the anion from a nonmetal that means atoms have a tendency to form a cation by losing its valence electron, this property is called electropositivity of an element.
- On the other hand an atom of a nonmetal has a tendency to form an anion by accepting electrons from outside into its valency shell. Ions have a stable electronic configuration of a noble gas.
| While going downwards in any group the electropositivity of elements goes on increasing while their electronegativity goes on decreasing.
While going from left to right in any period the electronegativity of elements goes on increasing while their electropositivity goes on decreasing. Larger the electropositivity or electro-negativity of the element higher the reactivity. |
The periodic trend in the metallic character of elements is clearly understood from their position in the modern periodic table. Therefore, the following trend is observed:
Gradation in Halogen Family: The group 17 contains the members of the halogen family. All of them have the general formula X2
A gradation is observed in their physical state down the group. Thus, fluorine (F2,) and chlorine (Cl2) are gases, bromine (Br2) in a liquid while iodine (I2) ll a solid.
Reaction of alkaline earth metal with water :
- A general chemical equation Indicating the reaction of alkaline earth metals is M + 2H2O -» M(OH)2 +H2.
- While going down the second group as Be → Mg → Ca → Sr → Ba,
- The gradation in this chemical property of the alkaline earth metals is observed.
- While going down the second group the reactivity of the alkaline earth metals goes on increasing and thereby the ease with which this reaction takes place also goes an increasing.
- Thus beryllium (Be) duel not react with water. Magnesium (Mg) reacts with steam, while calcium (Ca), strontium (Sr) and barium ( Ba ) react with water at room temperature with increasing rates.
Useful links :
| Main Page : - Maharashtra Board Class 10 Science & Technology Part-1,Part-2 - All chapters notes, solutions, videos, test, pdf.
Books : MSBSHSE -Class 10th Science Text Books – Chapter wise PDF for download Previous Chapter : Class 10-Sc. & Tech.-1-Chapter-1-Gravitation - Online Notes Next Chapter : Class 10-Sc. & Tech.-1-Chapter-3-Chemical reactions and equations - Online Notes |
Thank you for the wonderful notes and solution…it’s so good, easy to understand.. explaination is superb…
Co ni atomic mass number.?
Ok
Okay
Mam my question is plz 10th class part1 chapter 2nd periodic classification of elements short notes in test
Thanks
Thanks madam ☺️
Very superb notes 📝
I like his nots
Thank you 😊🙏🙏🙏🙏🙏🙏🙏🙏🙏🙏 your notes is so simple and good 💯😊 and easy to learn thank you so much fonce again 💯🙏😊
thanks man sirf
2025 exam
👏👍
Thanks for easy notes.
I proud of your notes and understanding easily.
Thanks for these note this will be very helpful for me in my boards
Its very easy
Thanks for easy notes
this notes is useful for students and teachers its so easy…
True 🔥
Thank you.I like this notes 🙏