Chemical Reactions and Equations
Based on Class 10-Science & Technology Part-1-Chapter-3- Maharashtra Board
Notes
|
Topics to be learn :
|
Introduction :
Elements and Compounds :
- Elements are divided into three classes i.e. metals, nonmetals and metalloids.
- When two or more elements combine chemically in a fixed proportion by weight, a compound is formed.
- The properties of a compound are altogether different from those of the constitutional elements.
Valency of element : The number of electrons that an atom of an element gives away or takes up while forming an ionic bond, is called the valency of that element.
Requirement for writing molecular formulae of different compounds and method of writing the molecular formulae of the compounds.
- While writing the molecular formulae of different compounds, the symbol of the radicals and their valence should be known.
- The number of the ions is written as subscript on the right of the symbol of the ion.
- By cross multiplication of valencies chemical formula is obtained.
Chemical Reaction :
Chemical Reaction : A process in which some substances undergo bond breaking and are transformed into new substances by formation of new bonds is called a chemical reaction.
- During chemical reactions composition of the matter changes and that change remains permanent and during physical change only the state of matter changes and this change is often temporary in nature.
Chemical equation :
Chemical equation : The representation of a chemical reaction in a condensed form using chemical formulae is called as the chemical equation.
Identify physical and chemical changes from the phenomena given in the following table. (Text Book)
Phenomenon
Physical change
Chemical change
(1) Transformation of ice into water
√
(2) Cooking of food
√
(3) Ripening of fruit
√
(4) Milk turned into curd
√
(5) Evaporation of water
√
(6) Digestion of food in the stomach
√
(7) Size reduction of naphtha balls exposed to air
√
(8) Staining of shahbadi or Kadappa tile by lemon juice
√
(9) Breaking of a glass object on falling from a height
√
Importance of a chemical reaction :
- Reactants are converted into products.
- Mass is conserved.
- Atoms are conserved.
- The properties and compositions of the products of a chemical reaction are different from those of its reactants.
- Generally, energy is either absorbed or evolved.
Writing a chemical equations :
Conventions used in writing a chemical equation:
- The reactants are written on the left hand side (LHS), while the products are written on the right hand side (RHS).
- Whenever there are two or more reactants, a plus sign ( + ) is written between each two of them. Similarly, if there are two or more products, a plus sign is written between each two of them.
- Reactant side and product side are connected with an arrow (→) pointing from reactants to products. The arrow represents the direction of the reaction. Heat is to be given from outside to the reaction, it is indicated by the Sign ∆ written above the arrow.
- The conditions like temperature, pressure, catalyst, etc., are mentioned above the arrow (→) pointing towards the product side.
- The physical states of the reactants and products are also mentioned in a chemical equation. The notations g, l, s, and aq are written in brackets as a subscript along with the symbols/formulae of reactants and products.
- The symbols and stand for gaseous, liquid, solid and aqueous respectively. If the product is gaseous, instead of (g) it can be indicated by an arrow ↑ pointing upwards. If the product formed is insoluble solid, then instead of (s) it can be indicated by an arrow ↓ pointing downwards.
- Special information or names of reactants/products are written below their formulae.
Balancing a Chemical Equation :
In a chemical reaction, the number of atoms of the elements in the reactants is same as the number of atoms of those elements in the product, such an equation is called a balanced equation.
- Example : AgNO3 + NaCl → AgCl + NaNO3
In the above reaction, the number of atoms of the elements in the reactants is same as the number of atoms of elements in the products.
Unbalanced equation :
- As per the law of conservation of mass, in any reaction, the total mass of each of the elements in the reactants is same as the total mass of each of the respective elements in the products.
- If the number of atoms of each element is not the same on the two sides of an equation, it is called an ‘unbalanced equation.
Steps in balancing a chemical reaction :
Consider the following equation as an example :
Sodium hydroxide + Sulphuric acid → Sodium sulphate + water.
Step 1 : Write the chemical equation from the given word equation. Or the given chemical equation as it is.
NaOH + H2SO4 → Na2SO4+ H2O ..............(1)
Stop 2 : Write the number of atoms of each element in the unbalanced equation on both sides of equations.
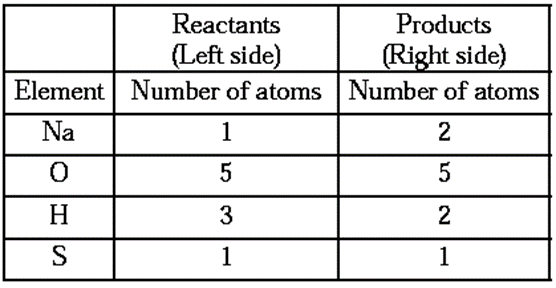
- It is seen that the number of atoms of all the elements on the two sides are not the same. It means that the equation (1) is not balanced.
- The number of oxygen and sulpher atoms on both sides of the equations are same, therefore equalize the number of sodium and hydrogen atoms.
Step 3 : To balance the number of sodium atoms
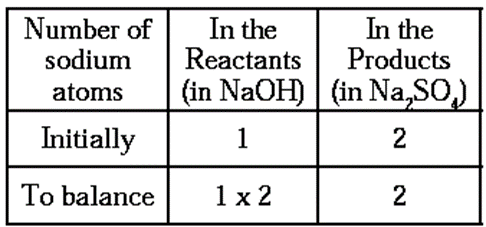
- To equalise the number of sodium atoms, we use 2 as the factor of NaOH in the reactants.
Now, the partly balanced equation becomes as follows :
2NaOH + H2SO4 → Na2SO4+ H2O ….(2)
- Check whether the equation (2) is balanced or not.
- We find that the equation (2) is not balanced, as the number of oxygen (6 and 5) and hydrogen atoms (4 and 2) are unequal on the two sides.
- First balance the hydrogen number as it requires a smaller factor.
Step 4 : Now, balance the number of hydrogen atoms :
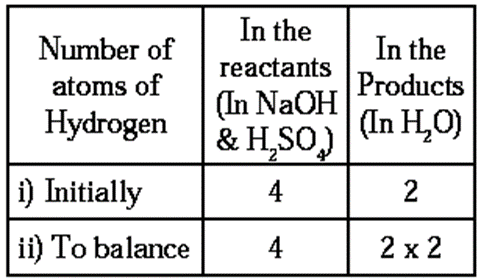
- To equalise the number of hydrogen atoms, we use 2 as the factor of H2O in the products. The equation then becomes
2NaOH + H2SO4 → Na2SO4+ 2H2O ….(3)
- Now, count the atoms of each element on both sides of the equation. The number of atoms on both sides are equal. Hence the balanced equation is
2NaOH + H2SO4 → Na2SO4+ 2H2O
- In this way, a balanced equation is obtained from an unbalanced equation by applying proper factors to appropriate reactant/product so as to balance the number of each element in steps.
Types of chemical reactions :
(1) Combination reaction: When two or more reactants combine in a reaction to form a single product, it is called a combination reaction.
Examples of combination reaction :
(1) The ammonia gas reacts with hydrogen chloride gas to form the salt in gaseous state, immediately it condenses at room temperature and gets transformed into the solid state.
NH3(g) + HCL(s) → NH4Cl(s)
(2) Magnesium burns in air to form white powder of magnesium oxide as a single product.
2Mg + O2 \(\underrightarrow{Heat}\) 2MgO
(2) Decomposition reaction : The chemical reaction in which two or more products are formed from a single reactant is called decomposition reaction.
Examples of decomposition :
(i) Thermal decomposition : The reaction in which a compound is decomposed by heating it to a high temperature is called thermal decomposition.
At high temperature, calcium carbonate decomposes into calcium oxide and carbon dioxide.
CaCO3(s) \(\underrightarrow{Heat}\) CacO(s) + CO2(g) ↑
(ii) Electrolytic decomposition : The reaction in which a compound is decomposed by passing an electric current through its solution or molten mass js called an electrolytic decomposition.
- When an electric current is passed through acidulated water, it is electrolyzed giving hydrogen and oxygen.
\(2H_2O(l) \underrightarrow{Electric\,\,current}2H_2↑+ O_2↑\)
(3) Displacement reaction : The reaction in which the place of the ion of a less reactive element in a compound is taken by another more reactive element by formation of its own ions, is called displacement reaction.
Examples of displacement reaction : (i) When zinc granules are added to the blue coloured copper sulphate solution, the zinc ions formed from zinc atoms take the place of Cu2+ ions in CuSO4 and copper atoms, formed from Cu2+ ions comes out i.e. the more reactive zinc displaces the less reactive Cu from copper sulphate. Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s) + Heat (ii) When iron powder is added to the blue coloured copper sulphate solution, the iron ions formed from iron atoms take the place of Cu2+ ions in CuSO4 and copper atoms, formed from Cu2+ ions comes out i.e. the more reactive iron displaces the less reactive Cu from copper sulphate. Fe(s) + CuSO4 → FeSO4(aq) + Cu(s) + Heat
(4) Double displacement reaction : The reaction in which the ions in the reactants are exchanged to form a precipitate are called double displacement reaction.
Examples of double displacement reaction : (1) Solutions of sodium chloride and silver nitrate react with each other forming a precipitate of silver chloride and a solution of sodium nitrate AgNO3 + NaCl → AgCl(s) + NaNO3 Silver Sodium Silver Sodium nitrate chloride chloride nitrate (Precipitate) White precipitate of AgCl is formed by exchange of ions Ag+ and Cl- between the reactants. (2) Barium sulphide reacts with zinc sulphate to form zinc sulphide and a white precipitate of barium sulphate. BaS + ZnSO4 → BaSO4(s) + ZnS Barium Zinc Barium Zinc sulphide sulphate sulphate sulphide (precipitate) White precipitate is formed by exchange of ions Ba++ and SO4-- between the reactants.
Endothermic and Exothermic Processes and Reaction :
Endothermic processes : In this process heat from outside is absorbed during some physical changes.
For example,
-
-
- Melting of ice
- Dissolution of potassium nitrate in water.
-
Endothermic reaction: During endothermic chemical reactions heat is either absorbed from the surroundings
- When KNO3(s) dissolves in water, there is absorption of heat during the reaction and the temperature of the solution falls.
KNO3(s) + H2O(l) + Heat → KNO3(aq)
Exothermic processes : In this process heat is given away during some physical changes.
For example,
-
-
- Formation of ice from water,
- Dissolution of sodium hydroxide in water.
-
Exothermic reaction: During exothermic chemical reactions heat is given away when reactants are transformed into the products,
- When NaOH(s) dissolves in water, there is evolution of heat leading to a rise in temperature.
NaOH(s) + H2O(l) → NaOH(aq) + Heat
Q- Is it possible to produce hydrogen by decomposition of water by mean of heat, electricity or light?
Yes, it is possible to produce hydrogen by decomposition of water by means of heat, electricity or light.
Rate of chemical reaction :
Some reactions are completed in short time, i.e. occur rapidly while some other require long time for completion, i.e. occur slowly. It means that the rate of different reaction is different.
- One or more chemical reactions take place during every chemical change.
- Strong acid and _ strong base react instantaneously.
- In our body, enzymes increase the rate of physiological reactions.
- If the rate of the chemical reaction is fast, it is profitable for the chemical factories.
- The rate of chemical reaction is important with respect to environment.
- The ozone layer in the earth's atmosphere protects the life on earth from the ultraviolet radiation of the sun. The process of depletion or maintenance of this layer depends upon the rate of production or destruction of ozone molecules.
Factors affecting the rate of a chemical reaction : (1) Nature of Reactants : Both Al and Zn reacts with dilute hydrochloric acid, H2 gas is liberated and water soluble salts of these metals are formed. However, aluminium metal reacts faster with dil.HCl as compared to zinc metal. Al is more reactive than Zn. Therefore, the rate of reaction of Al with hydrochloric acid is higher than that of Zn. Nature or reactivity of reactants influences the rate of a chemical reaction. (2) Size of the particles of reactants : In the reaction of dil HCl and Shahabad tile, CO2 effervescence is formed slowly. On the other hand, CO2 effervescence forms at faster speed with the powder of Shahabad tile. (3) Concentration of the reactants : In the reaction of dil. HCl and CaCO3, CaCO3 disappears slowly and CO2 also liberates slowly. On the other hand the reaction with concentrated HCl takes place rapidly and CaCO3 disappears fast. Concentrated acid reacts faster than dilute acid, that means the rate of a reaction is proportional to the concentration of reactants. (4) Temperature of the reaction : Lime stone on heating decomposes to give CO2, which turns lime water milky. On the other hand, the lime water does not turn milky before heating the lime stone; because of the zero rate of reaction. The above observation indicates that the rate of a reaction increases on increasing the temperature. (5) Catalyst : On heating potassium chlorate (KCIO3) decomposes into potassium chloride and oxygen Slowly. 2KCIO3 \(\underrightarrow{Δ}\) 2KCl + 3O2
Oxidation and Reduction :
Oxidation : The chemical reaction in which a reactant combines with oxygen or loses hydrogen to form the product is called oxidation reaction. The chemical substances which bring about an oxidation reaction by making oxygen available are called oxidants or oxidizing agents.
In the combustion of carbon, oxygen is an oxidant.
Examples :
C +O2 \(\underrightarrow{Δ}\) CO2 ↑
CH3 – CH3 \(\underrightarrow{Δ}\) CH2 =CH2 + H2 ↑
A variety of oxidants : K2Cr207/H2SO4, KMnO4/ H2SO4 are the commonly used chemical oxidants. Hydrogen peroxide (H2O2) is used as a mild oxidant. Ozone (O3) is also a chemical oxidant. Nascent oxygen is generated by chemical oxidants and it is used for the oxidation reaction. O3 → O2 + [O] H2O2 → H2O + [O] K2Cr2O2 + 4H2SO4 → K2SO4 + Cr2(SO4)3 + 4H2O + 3[O] 2KMnO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 3H2O + 5[O] Nascent oxygen is a state prior to the formation of the O2 molecule. It is the reactive form of oxygen and is represented by writing the symbol as [O].
Reduction : The chemical reaction in which a reactant gains hydrogen or loses oxygen to form the product is called reduction. The chemical substance that brings about reduction is called a reductant, or a reducing agent.
When hydrogen gas is passed over black copper oxide a reddish coloured layer of copper is formed.
CuO + H2 → Cu + H2O
Redox reaction : The reaction which involves simultaneous oxidation and reduction is called an oxidation-reduction or redox reaction. In a redox reaction, one reactant gets oxidised while the other gets reduced during a reaction. Redox reaction = Reduction + Oxidation In redox reaction, the reductant is oxidizedby the oxidant and the oxidant is reduced by the reductant. Examples: CuO(s) + H2(g) → Cu(s) + H2O(g) 2H2S + SO2 → 3S + 2H2O MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
Corrosion :
Due to various components of atmosphere, oxidation of metals takes place, consequently resulting in their damage. This is called ‘corrosion.
Iron rusts and a reddish coloured layer is collected on it. This is corrosion of iron. This is also termed as rusting of iron. Its formula is Fe2O3.H20.
Prevention of corrosion : Corrosion damages buildings, bridges, automobiles, ships, iron railings and other articles made of iron. It can be prevented by using an anti-rust solution, coating surface by the paint by processes like galvanising and electroplating with other metals.
Rancidity :
Fats and oils in food, is kept for a long time, gets oxidised, it is found to have foul odour called rancidity.
Useful links :
| Main Page : - Maharashtra Board Class 10 Science & Technology Part-1,Part-2 - All chapters notes, solutions, videos, test, pdf.
Books : MSBSHSE -Class 10th Science Text Books – Chapter wise PDF for download Previous Chapter : Class 10-Sc. & Tech.-1-Chapter-2-Periodic Classification of Element - Online Notes Next Chapter : Class 10-Sc. & Tech.-1-Chapter-4-Effects of electric current - Online Notes |

Please I want short answers for science 1
thanks
Nice ans I have got good marks but I want Little bit short answer