Heat
Based on Class 10-Science & Technology Part-1-Chapter-5- Maharashtra Board-Audio-Text Notes, Videos, PDF
Notes
Difference between heat and temperature :
Heat is a form of energy. Particles of Matter (atoms, molecules, etc.) possess potential energy and kinetic energy.
Total energy (potential energy + kinetic energy) of all particles of matter in a given sample is called its thermal energy.
When two bodies at different temperatures are in thermal contact with each other, there is transfer of thermal energy from a body at higher temperature to a body at lower temperature. This energy in transfer is called heat.
It is expressed in joule, calorie and erg.
Temperature :
Temperature is a quantitative measure of degree of hotness or coldness of a body. It is expressed in °C, °F or K (Kelvin). Temperature determines the direction of energy transfer.
Different ways of heat transfer: Conduction, Convection and Radiation.
Latent heat : When there is a change of state of a substance,
- solid ↔ liquid,
- liquid ↔ gas (vapour),
- solid ↔ gas (vapour)
Heat energy is absorbed by the substance or heat energy is removed from the substance at constant temperature.
This heat energy is called latent heat of transition (or transformation or change of State).
Latent heat per unit mass of the substance is called specific latent heat.
The amount of heat energy absorbed at constant temperature by unit mass of a solid to convert into liquid phase is called the specific latent heat of fusion, and that constant temperature is called the melting point of the substance.
The amount of heat energy absorbed at constant temperature by unit mass of a liquid to convert into gaseous phase is called the specific latent heat of vaporization and that constant temperature is called the boiling point of the substance.
Specific latent heat is expressed in J/kg, erg/g, cal/g, kJ/kg and kcal/ kg. [/responsivevoice]
At a pressure of one atmosphere
| Specific latent heat of
fusion (L) |
Specific latent heat of
Vaporisation (L) |
|||||
| Substance | Melting Point °C | Boiling Point °C | kJ/kg | cal/g | kJ/kg | cal/g |
| Water/Ice | 0 | 100 | 333 | 80 | 2206 | 540 |
| Copper | 1083 | 2062 | 134 | 49
|
5060 | 1212 |
| Ethyl alcohol | 117 | 78 | 104 | 26 | 8540 | 200 |
| Gold | 1063 | 2700 | 144 | 15.3 | 1580 | 392 |
| Silver | 962 | 2162 | 88.2 | 25 | 2330 | 564 |
| Lead | 327.5 | 1749 | 26.2 | 5.9 | 859 | 207 |
Heat absorbed or given out in change of state = mL, ( m is the mass of the substance )
[responsivevoice voice="UK English Female" rate="0.8" pitch="0.8" buttontext="Listen to this"]
Boiling point of a liquid: The constant temperature at which a liquid transforms into gaseous state is called the boiling point of the liquid.
(Note : On application of pressure, the boiling point of a liquid is raised. On reducing the pressure, the boiling point is lowered)
Regelation: The phenomenon in which ice converts to liquid due to applied pressure and then re-converts to ice once the pressure is removed is called regelation.
Anomalous behaviour of water: If water is heated from 0°C to 4°C, it contracts instead of expanding. At 4°C its volume is minimum. If the water is heated further, it expands, i.e., its volume increases. This abnormal behaviour of water in the temperature range 0°C to 4°C is called anomalous behaviour of water. The density of water is maximum at 4°C.
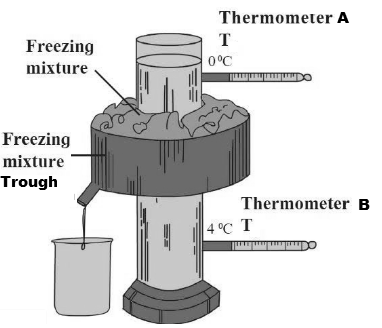
Hope‘s apparatus is used to study anomalous behaviour of water.
Normally, liquids contract on cooling & the density increases. but, water is special. .
Anomalous expansion of water is an abnormal property of water whereby it expands instead of contracting when the temperature goes from 4°C to 0°C, and it becomes less dense
Hope's apparatus consists of a vertical vessel full of water surrounded round the middle by a trough of cooling ice. Two thermometers, one above and one below the trough, measure the temperature of the water. It is designed to demonstrate that water reaches its maximum density at 4 °C (39 °F).
In first Phase, the water temperature is maintained higher than 4°C, and the cylindrical vessel is filled with this water.
- The reading of the two thermometers is noted.
- Temperature in A & B is observed to be the same in the beginning.
- Then freezing mixture containing ice and salt is added to the trough and after some time (say 30 minutes) temperature is noted.
- In this case, the temperature of the water in the lower part of the vessel below the trough is found to be less as compared to the upper part.
- Due to the addition of freezing mixture, decreased the temperature of the water and due to an increase in density (above 4°C) the water at lower temperature sinks down resulting in a lowering of the temperature at thermometer B as compared to the temperature shown at thermometer A.
In the second phase, the water is maintained to a temperature near to 4°C and filled in the cylinder.
- After the addition of the freezing mixture, the temperature of water drops below 4°C and it begins to expand thus comes to the upper part (ice floats on water).
- Thus, the temperature of the upper part of the vessel decreases resulting in a lowering of the temperature at thermometer A as compared to the temperature at thermometer B.
Conclusion : When water at 0 ºC is heated, it contracts till 4 ºC, instead of expanding. On further heating from 4 ºC, water expands like other liquids do. The special behaviour of water between 0ºC and 4ºC is called the anomalous behaviour of water
When the water bodies (such as lakes; rivers; seas;), freeze in cold countries in winter, only the upper surface freezes to form ice. Water below ice stays at 4ºC and hence, water animals can survive. [/responsivevoice]
Watch Video : [video_lightbox_youtube video_id="Zh45_WT8Cg0&rel=0" width="640" height="520" start="10" anchor="Hope's Apparatus"]
[responsivevoice voice="UK English Female" rate="0.8" pitch="0.8" buttontext="Listen to this"]
Dew point temperature : If the temperature of unsaturated air is decreased, a temperature is reached at which the air becomes saturated with water vapour. This temperature is called the dew point temperature.
Humidity : The moisture, i.e., the presence of water vapour, in the atmosphere is called humidity.
Absolute humidity : The mass of water vapour present in a unit volume of air is called absolute humidity. Generally it is expressed in kg/m.
Relative humidity : The ratio of the actual mass of water vapour content in the air for a given volume and temperature to that required to make the same volume of air saturated with water vapour at the same temperature is called the relative humidity. This ratio, multiplied by 100, gives the percentage relative humidity. At the dew point the relative humidity is 100%.
Unsaturated with water vapour: When air contains water vapour less than its capacity to hold water vapour at that temperature, it is said to be unsaturated with water vapour.
Unit of heat : Heat can be expressed in various units, e.g., joule (J), erg, calorie (cal), kilocalorie (kcal).
The amount of heat necessary to raise the temperature of 1 g of water by 1°C from 14.5 °C to 15.5 °C is called one calorie.
The amount of heat necessary to raise the temperature of 1 kg of water by 1°C from 14.5 °C to : 15.5 °C is called one kilocalorie.
1 kcal = 103 cal, 1 cal=4.18 J,
1 kcal = 4.18 x 103J. [/responsivevoice]
[responsivevoice voice="UK English Female" rate="0.8" pitch="0.8" buttontext="Listen to this"]
Specific heat capacity (c) : The amount of heat energy required to raise the temperature of a unit mass of an object by 1°C is called the specific heat capacity or simply specific heat of the object. It is expressed in various units such as J/kg.°C, erg/g.°C. cal/g.°C, kcal/kg.°C.
Substance & Spacific heat (c) in cal/g.°C
Water : 1.0 cal/g.°C
Paraffin : 0.54 cal/g.°C
Kerosene : 0.52 cal/g.°C
Aluminium : 0.215 cal/g.°C
Iron : 0.110 cal/g.°C
Copper : 0.095 cal/g.°C
Silver : 0.056 cal/g.°C
Mercury : 0.033 cal/g.°C
Heat absorbed by an object = mc∆T, where m is the mass of the object and ∆T is the increase in the temperature of the object.
Heat lost (given out) by an object = mc∆T
Here ∆T is the decrease in the temperature of the object.
Specific latent of fusion of ice is 80 cal/g. Explain this statement.
Ans. When 1 g of ice at a pressure of one atmosphere and at a temperature 0°C is converted into 1 g of water, heat absorbed by the ice is 80 cal.
The specific latent heat of fusion of silver is 88.2 kJ/kg. Explain this statement.
Ans. When 1 kg of silver at a pressure of one atmosphere and at a temperature of 962°C (melting point of silver) is converted into 1 kg of silver in liquid phase, heat absorbed by the silver is 88.2 kJ.
The specific latent heat of vaporization of water is 540 cal/g. Explain this statement.
Ans. When 1 g of water at a pressure of one atmosphere and at a temperature of 100°C is converted into 1 g of steam, heat absorbed by the water is 540 cal.
Principle of heat exchange : If a system of two objects is isolated from the environment by keeping it inside a heat resistant box, then no energy can leave the box or enter the box.
In this situation, heat energy lost by the hot object = heat energy gained by the cold object.
In due course, the two objects attain the same temperature.
Explanation of the principle of heat exchange.
Consider two objects A and B at different temperature T1, and T2, respectively are enclosed in a box of heat resistant material as shown in fig.
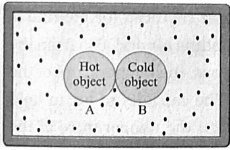
Let m1 = mass of A,
m2=mass of B,
c1=specific heat capacity of A,
c2=specific heat capacity of B and
T = common temperature attained by A and B after the heat exchange between A and B.
Please note here, no heat leaves the box or enters the box from outside.
Hence, if T1 > T2,
heat energy lost by A (Q1) = heat energy gained by B (Q2).
m1c1 (T1-T) =m2c2 (T-T2)
If m1,c1, T1, T, m2 and T2 are known, c2 can be calculate.
Sublimation: The phenomenon in which a solid directly passes to the gaseous state without passing through the intermediate liquid state is known as sublimation.
Iodine, ammonia and camphor possess the property of sublimation.
Effect of pressure on the melting point of a substance : At a given pressure, a given solid melts at a fixed temperature. A change in pressure changes the melting point of the substance. In the case of the solids which expand on melting, an increase in the pressure raises the melting point of the solid, e.g., lead and wax.
In the case of the solids which contract on melting, an increase in pressure lowers the melting point of the solid, e.g., ice, antimony and bismuth.
Effect of pressure on the boiling point of a liquid : The boiling point of a liquid depends on the pressure on its surface. An increase in pressure raises the boiling point of a liquid while a decrease in pressure lowers its boiling point. [/responsivevoice]
Important Formulae :
Q=amount of heat required, m=mass, L=Latent heat, c=specific heat
Latent heat of melting ice=80 cal/g
Latent heat of water =540 cal/g
Specific heat of water =1 cal/g.0C
Q=mL
Q=mc(T2-T1)+mL
m1c(T1-T)=m2c(T-T2)
Q=mc∆T
Click on below link to get PDF from store
MSBSHSE-Class 10-Science & Technology-1-Chapter-5-Heat-Notes
Useful links :
| Main Page : - Maharashtra Board Class 10 Science & Technology Part-1,Part-2 - All chapters notes, solutions, videos, test, pdf,
Books : MSBSHSE -Class 10th Science Text Books – Chapter wise PDF for download Videos : Class 10th Videos of all chapters Previous Chapter : Chapter 4: Effects of Electric Current - Online Notes Next Chapter :Chapter 6. Refraction of light - Online Notes |

Very good to understand without textbook we know all the concepts perfectly.
Important points is without textbook
hi sweet
Very useful 👌 keep doing
Very best notes you are giving!! Thankuu
Very simple and important notes
Yes very simple notes