Carbon Compound
Class 10-Science & Technology Part-1-Chapter-9- Maharashtra Board
Notes
|
Topics to be learn :
|
Compounds :
- Organic and inorganic compounds are the two important types of compounds.
- Objects in everyday we use such as foodstuff, fibres, paper, medicines, wood, fuels are made of various compounds. The constituent elements common in these compounds are carbon (C), hydrogen (H) and oxygen (O).
- The element carbon belongs to group IV in the periodic table and its electronic configuration is 2,4. The valency of carbon is 4.
Chemical bond : The force of attraction which holds the atoms together in a molecule is called a chemical bond.
- The number of chemical bonds that an atom of an element forms is called catenation power.
- The two important types of chemical bonds are ionic bonds and covalent bonds.
Bonds in Carbon compounds :
Properties of carbon compounds :
- Carbon compounds have low melting and boiling points.
- These compounds are bad conductors of heat and electricity.
- The chemical bonds in carbon compounds do not produce ions.
Background of bond formation by carbon :
| Carbon atom | Electronic Configuration | Number of electron in the Valence shell | Nearby noble gas and the electronic configuration | |
|
6C |
2.4 |
4 |
He | Ne |
| 2 | 2.8 | |||
Carbon and Neon Configuration :
- Carbon atoms share four valence electrons to attain neon configuration.
- Shared electrons are accommodated in overlapping regions of valence shells, resulting in a noble gas configuration.
- This route ensures atoms remain electrically neutral, promoting stability.
- Therefore, this route is adopted by carbon atom to attain a noble gas configuration.
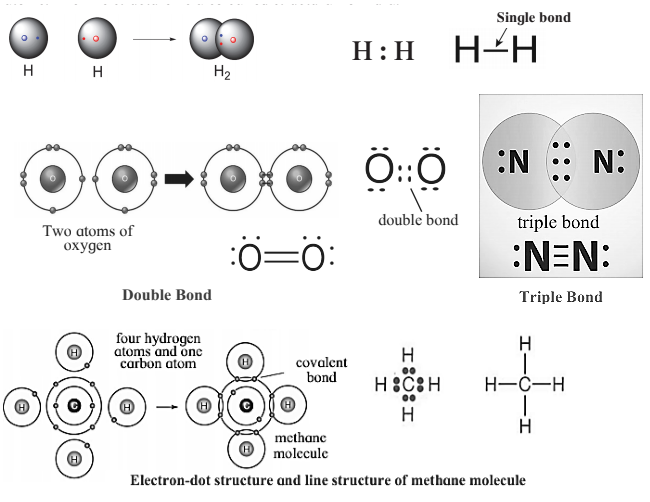
Covalent Bond :
- A chemical bond formed by sharing two valence electrons between two atoms is called a covalent bond.
- Covalent bond is represented by an electron-dot structure, with a circle around the atomic symbol and each valence electron indicated by a dot or cross.
- One covalent bond constitutes one pair of shared electrons.
- A small line joining the symbols of the two atoms is also represented by a covalent bond.
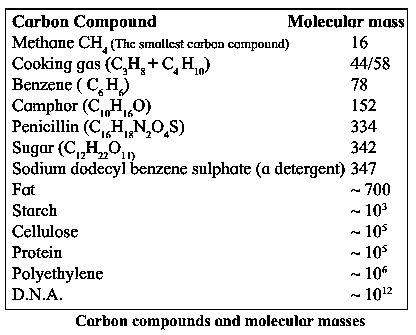
Carbon : A Versatile Element :
- The entire living kingdom is made from carbon.
- Carbon is the basic ingredient of our body.
- Millions of molecules ranging from the small methane molecule to the extremely big D.N.A. molecule are made from carbon.
- The molecular masses of carbon compounds range up to 1012
Characteristics of carbon :
Characteristics of carbon :
(i) Catenation power : Carbon is characterized by its unique ability to form strong covalent bonds with other carbon atoms, resulting in the formation of big molecules. This property is known as catenation power, which is the ability of carbon atoms to form multiple bonds or single bonds. Carbon compounds can be open or closed chains, with open chains being straight or branched, and closed chains being ring structures.
(ii) Ability of carbon atoms to form multiple bonds as well as single bonds : Carbon can form multiple bonds, such as single, double, or triple covalent bonds, which increases the number of carbon compounds.
- For example, there are three compounds, namely, ethane (CH3-CH3), ethene (CH2=CH2) and ethyne (CH ≡ CH) which contain two carbon atoms.
(iii) Can form bonds with four other atoms : Tetravalent carbon allows one carbon atom to form bonds with four other atoms, resulting in many compounds with different properties depending on the atoms to which carbon is bonded.
- For example, five different compounds can be formed using one carbon atom and two monovalent elements, such as hydrogen and chlorine.
- Carbon atoms can also form covalent bonds with elements like oxygen, nitrogen, sulfur, and halogen, forming a large number of carbon compounds.
(4) Isomerism : Isomerism is one more characteristic of carbon compound which is responsible for large number of carbon compounds.
Hydrocarbons : Saturated and Unsaturated :
Saturated hydrocarbon : In hydrocarbon, the four valencies of carbon atom are satisfied only by the single bonds, such compounds are called saturated hydrocarbons.
Examples : Methane (CH4), Ethane (C2H6), Propane (C3H8).
(i) Methane molecule contains only one carbon atom. In methane, four hydrogen atoms are bonded to carbon atom by four covalent bonds.
(ii) Ethane : In ethane, Join the two carbon atoms with single bonds C – C. Use the 6 hydrogen atoms in the molecular formula for fulfilling the tetravalency of both the carbon atoms.
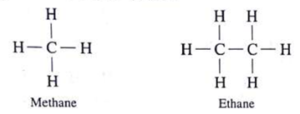
Unsaturated hydrocarbon : The carbon compounds having a double bond or triple bond between two carbon atoms are called unsaturated hydrocarbons.
- The unsaturated hydrocarbons containing a carbon-carbon double bond are called alkenes. e.g. Ethene (CH2 = CH2), Propene (CH3-CH = CH2)
- The unsaturated hydrocarbons containing a carbon-carbon triple bond are called alkynes. e.g. Ethyne (CH ≡ CH)
Straight chains, Branched chains and Rings of Carbon atoms :
The carbon skeleton determines the shape of the molecule of a carbon compound.
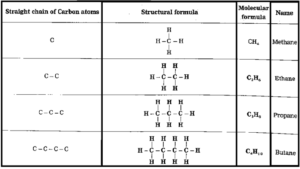
A straight chain of carbon atoms is formed by joining the carbon atoms are next to the other.
The first column of the table shows straight chains of carbon atoms, the structural formulae of the corresponding straight chain hydrocarbons are shown in the second column satisfying the tetravalency of the carbon atom by joining them to hydrogen atoms.
Structural Isomerism : Butane is represented by two different compounds, as their structural formulae are different. These two different structural formulae have the same molecular formula, i.e. C4H10.
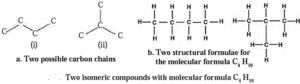
The carbon chain (i) in the figure (a) is a straight chain of carbon atoms, whereas the carbon chain (ii) is a branched chain of carbon atoms.
Closed chain : The closed chain of carbon atoms are present in some carbon compounds, wherein, rings of carbon atoms form. For example, the molecular formula of cyclohexane is C6H12 and its structural formula contains a ring of six carbon atoms.
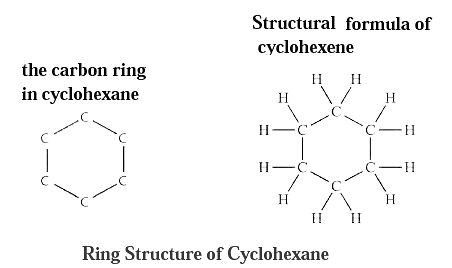
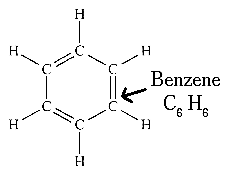
Aromatic compounds : Benzene is a cyclic unsaturated hydrocarbon. There are three alternate double bonds in the six membered ring structure of benzene. The compounds having this characteristic unit in their structure are called aromatic compounds.
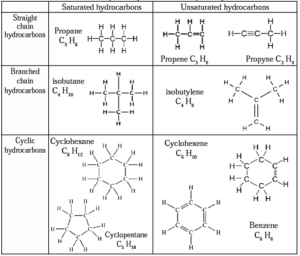
All types of carbon compounds whether straight chain, branched chain or cyclic, can be saturated or unsaturated. This is explained by the various examples of hydrocarbons in below table :
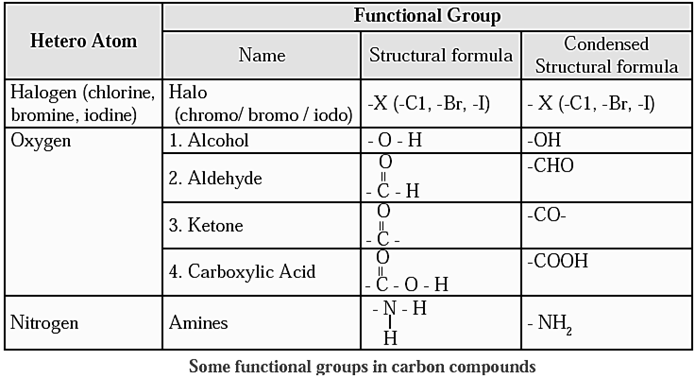
Functional Groups in Carbon Compounds
Functional group: The compound acquire specific chemical properties due to these hetero atoms or the groups of atoms that contain hetero atoms, irrespective of the length and nature of the carbon chain in that compound. Therefore, these hetero atoms or the groups of atoms containing hetero atoms are called functional groups.
Some functional groups in carbon compounds :
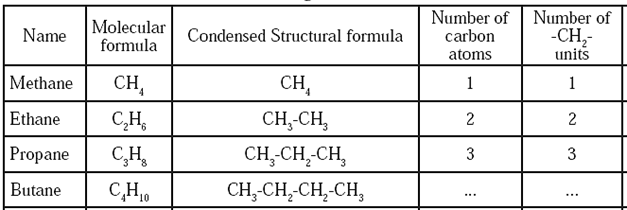
Homologous series :
Homologous series refers to a sequence of carbon compounds with different lengths but similar chemical properties due to the presence of the same functional group. These compounds are formed by joining the same group in place of a hydrogen atom on chains with sequentially increasing length.
Two adjacent members of the series differ by only one —CH2— (methylene) unit and their mass differ by 14 units.
Example : The homologous series of straight chain alkanes can be represented by the general formula CnH2n+2, The members of this series are as follows :
Characteristics of Homologous series :
(1) In homologous series while going in an increasing order of the length of carbon chain.
- one methylene unit ( — CH2 — ) gets added
- molecular mass increases by 14u
- number of carbon atoms increases by one.
(2) Chemical properties of members of a homologous series show similarity due to the presence of the same functional group in them.
(3) Each member of the homologous series can be represented by the same general molecular formula.
(4) While going in an increasing order of the length there is gradation in the physical properties, i.e. the boiling and melting points.
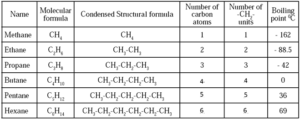
Some Homologous Series :
Some Homologous Series :
(a) Homologous Series of Alkanes :
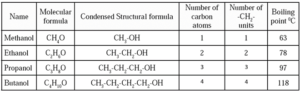
(b) Homologous Series of Alcohols :
(c) Homologous Series of Alkenes :
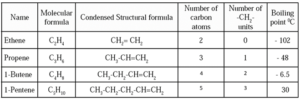
Nomenclature of Carbon compounds :
IUPAC nomenclature system :
International Union for Pure and Applied Chemistry (IUPAC) put forth a nomenclature system based on the structure of the compounds, and it was accepted all over the world. There are three units in the IUPAC name of any carbon compound: parent, suffix and prefix. These are arranged in the name as follows :
Prefix — parent — suffix : An IUPAC name is given to a compound on the basis of the name of its parent alkane. The name of the compound constructed by attaching appropriate suffix and prefix to the name of the parent alkane.
The steps in the IUPAC nomenclature of straight chain compounds are as follows :
Step 1: To determine the parent alkane of a straight chain compound, draw its structural formula and count the number of carbon atoms. If the compound has a double bond, change the ending of the parent name from 'ane' to 'ene', and if it has a triple bond, change the parent name from 'ane' to 'yne'. This helps identify the alkane with the same number of carbon atoms.
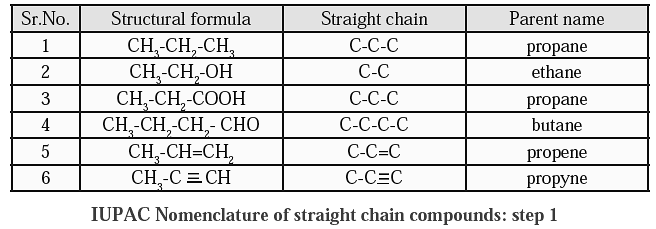
Step 2: If the structural formula contains a functional group replace the last letter ‘e’ from the parent name by the condensed name of the functional group as the suffix. (Exception : The condensed name of the functional group ‘halogen’ is always attached as the prefix. )
IUPAC Nomenclature : Step- 2 :

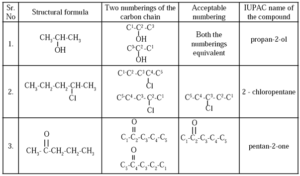
Step 3: It involves naming carbon atoms in a carbon chain, assigning a number to the carbon in the functional group (CHO or COOH) if present, or a chain in two directions. The final name should include a digit and a character separated by a small horizontal line.
IUPAC Nomenclature : Step- 3 :
Chemical Properties of Carbon Compounds :
(1) Combustion : Methane undergoes combustion in the presence of oxygen to emit heat and light to form carbon dioxide and water.
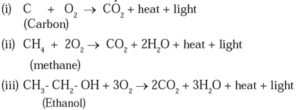
(2) Oxidation : Ethanol gets oxidized in the presence of alkaline potassium permanganate to form ethanoic acid.
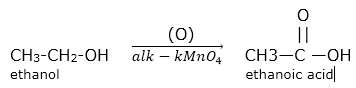
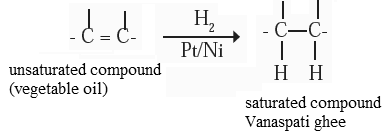
(3) Addition reaction : Vegetable oil (unsaturated compound) undergoes addition reaction with hydrogen in the presence of nickel catalyst to form vanaspati ghee (saturated compound).
(4) Substitution reaction: The saturated hydrocarbons are not reactive as the single bonds C-H and C-C are very strong. However, saturated hydrocarbons, in presence of sunlight react rapidly with chlorine. In this reaction chlorine atoms replace, one by one, all the hydrogen atoms in the saturated hydrocarbon. The reaction in which the place of one type of atom/group in a reactant is taken by another atom/group of atoms, is called substitution reaction. Chlorination of methane, is a substitution reaction which gives four products.
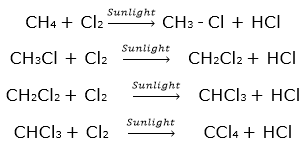
- Still larger number of products are formed in chlorination reaction of higher homologues of alkanes.
[/spoiler]
Important carbon compounds : Ethanol and Ethanoic Acid
Ethanol :
- Ethanol is a colourless liquid at room temperature and its boiling point is 78 °C.
- Generally ethanol is called alcohol or spirit.
- Ethanol is soluble in water in all proportions.
- When aqueous solution of ethanol is tested with litmus paper it is found to be neutral.
- Ethanol being good solvent, it is used in medicines such as tincture iodine (solution of iodine and ethanol), cough mixture and also in many tonics.
Methanol (CH3OH) :
- The lower homologue of ethanol, is poisonous, and intake of its small quantity can affect vision and at times can be lethal.
- To prevent the misuse of the important commercial solvent ethanol, it is mixed with the poisonous methanol. Such ethanol is called denatured spirit. A blue dye is also added to it, so that it is easily recognised.
Chemical properties of ethanol :
Chemical properties of ethanol :
(i) Reaction with sodium : All the alcohols react with sodium metal to liberate hydrogen gas and form sodium alkoxide salts. When ethanol reacts with sodium metal, hydrogen gas and sodium ethaoxide are formed.
![]()
(ii) Dehydration reaction: When ethanol is heated at the temperature 170 °C with excess amount of concentrated sulphuric acid, to form ethene, with elimination of water molecule.
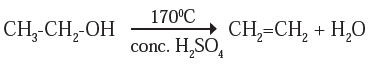
Here, concentrated sulphuric acid acts as adehydrating agent.
Ethanoic acid :
- Ethanoic acid is a colourless liquid with boiling point 118 °C.
- Ethanoic acid is commonly known as acetic acid.
- It’s aqueous solution is acidic and turns blue litmus red.
- Vinegar, which is used as preservative in pickles, is a 5-8% aqueous solution of acetic acid.
- The melting point of pure ethanoic acid is 17 °C. Therefore during winter in cold countries ethanoic acid freezes at room temperature itself and looks like ice. Therefore it is named ‘glacial acetic acid’.
Chemical Properties of ethanoic Acid :
Chemical Properties of ethanoic Acid :
(i) Reaction with base :
A reaction with strong base : Ethanoic acid gives neutralization reaction with a strong base sodium hydroxide to form a salt and water.
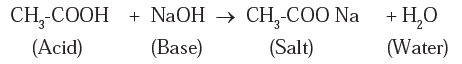
Reaction with carbonate and bicarbonate : Ethanoic acid reacts with sodium carbonate to form sodium ethanoate, water and carbon dioxide.
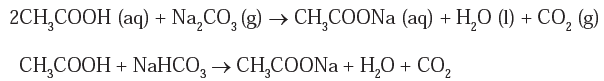
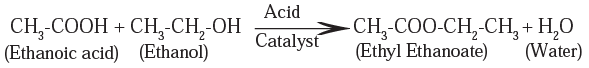
(ii) Esterification reaction : Ethanoic acid reacts with ethanol in presence of an acid catalyst, ethyl ethanoate (ester) is formed. This reaction is known as esterification.
Saponification : When an ester is reacted with the alkali sodium hydroxide, the corresponding alcohol and sodium salt of carboxyclic acid are obtained. This reaction is called saponification reaction, as it is used for preparation of soap from fats.
Ester + Sodium hydroxide ---> sodium Carboxylate + Alcohol
- When fat is heated with NaOH solution, soap and glycerin are obtained.
Macromolecules and Polymers :
Macromolecules : The giant carbon molecules formed from hundreds of thousands of atoms are called macromolecules.
- Natural macro-molecules : Polysaccharides, proteins and nucleic acid and rubber, etc.
- Manmade macromolecules: Elastomers, plastic, nylon, etc.
Polymers: A macromolecule formed by regular repetition of a small unit is called polymer. The small unit that repeats regularly to form a polymer is called monomer. The reaction by which monomer molecules are converted into a polymer is called polymerization.

Various polymers and their uses :
Various polymers and their uses :

- The polymers in the above examples are formed by repetition of single monomer. These are called homopolymers.
- The other type of polymers are formed from two or more monomers. They are called copolymers. For example, PET is poly ethylene terephthalate.
- The structures of polymers are linear as in the above examples or they are branched and cross linked as well.
- Polymers aquire various properties as per the nature of the monomers and the type of structure.
Some Natural Polymers and their occurrence :
Some Natural Polymers and their occurrence :
| Polymer | Name of the monomer | Occurrence |
| Polysaccharide | Glucose | Starch
|
| Cellulose | Glucose | Wood (cell walls of plant cells) |
| Proteins | Alpha amino acids | Muscles, hair, enzymes, skin, egg |
| D.N.A. | Nucleotide (deoxyribosephosphate) | Chromosomes of organisms |
| R.N.A. | Nucleotide (ribosephosphate ) | Nucleus and cytoplasm of cell |
| Rubber | 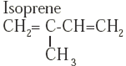 |
Latex of rubber tree |
Useful links :
| Main Page : - Maharashtra Board Class 10 Science & Technology Part-1,Part-2 - All chapters notes, solutions, videos, test, pdf.
Books : MSBSHSE -Class 10th Science Text Books – Chapter wise PDF for download Previous Chapter : Chapter 8- Metallurgy - Online Notes Next Chapter : Chapter 10 -Space Missions - Online Notes |

It’s very helpful for study
Nice 👍 , but try to make the notes in flow chart, it might easier to understand any topic. Please work on it
Thanks for your valuable suggestion.
Yes you are right 👍
notes something that childrens use as revision so for more understanding make it short use diffrent kind of colours pictures flowcharts and some kind of animation to make short like write summary
Noted, thanks for your valuable suggestions
Very nice notes.I always used