Chemical Reactions and Equations
NCERT-Class 10-Science-Chapter-1
Notes
|
Topics to be learn : Chemical reactions:
Types of chemical reactions:
|
Chemical Reaction : A process in which some substances undergo bond breaking and are transformed into new substances by formation of new bonds is called a chemical reaction.
Chemical equation :
A chemical equation is an expression for given chemical change in terms of symbols or formulae of the reactants and products.
Example :The reaction of zinc with dilute sulphuric acid to produce zinc sulphate and hydrogen is given by the following chemical equation:
Zn + H2SO4 —-> ZnSO4 + H2.
Changes in Chemical Reactions : When a chemical reaction occurs, one or more of the following changes take place
- Change in state
- Change in colour
- Evolution of a gas
- Evolution or absorption of heat (change in temperature).
Examples :
- Burning of magnesium ribbon in air : Burning of magnesium ribbon in air gives a powder of MgO. So there is a change of state with formation of new substance and heat is produced.
- Addition of lead nitrate solution to potassium iodide solution : Addition of colourless lead nitrate solution to potassium iodide solution gives yellow coloured precipitate (lead iodide). So there is a change of colour.
- Addition of dilute hydrochloric acid to zinc granules : Addition of dilute hydrochloric acid to zinc granules gives a gas (H2) with effervescence and heat is evolved. There is change in temperature.
Balanced chemical equation :
In a chemical reaction, the number of atoms of the elements in the reactants is same as the number of atoms of those elements in the product, such an equation is called a balanced equation.
- Law of conservation of mass to be kept in mind while we balance a chemical equation.
- According to law of conservation of mass matter can neither be created nor destroyed.
- Thus during a chemical reaction the total mass of the reactants and products remains the same.
- Therefore, for a complete chemical equation, the number of atoms of various elements on both sides are made equal, i.e., the equation is balanced.
Example : AgNO3 + NaCl —> AgCl + NaNO3
- In the above reaction, the number of atoms of the elements in the reactants is same as the number of atoms of elements in the products.
Writing of skeletal equation :
Q. Write the skeletal equation for the following reactions:
(i) Hydrogen sulphide reacts with sulphur dioxide to form sulphur and water.
(ii) Methane on burning combines with oxygen to produce carbon dioxide and water. (CBSE 2012)
Ans. (i) H2S + SO2 ——> S + H2O
(ii) CH4 + O2 ——> CO2 + H2O
[collapse]
Steps in balancing a chemical reaction :
Consider the following equations as an example :
Example 1:
Sodium hydroxide + Sulphuric acid à Sodium sulphate + water.
Step 1 : Write the skeletal equation from the given word equation. Or the given chemical equation as it is.
NaOH + H2SO4 ——> Na2SO4+ H2O ..............(1)
Stop 2 : Write the number of atoms of each element in the unbalanced equation on both sides of equations.
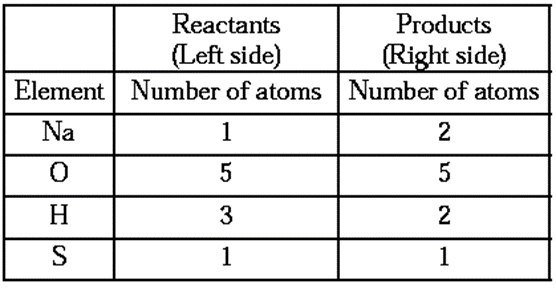
It is seen that the number of atoms of all the elements on the two sides are not the same. It means that the equation (1) is not balanced.
The number of oxygen and sulpher atoms on both sides of the equations are same, therefore equalize the number of sodium and hydrogen atoms.
Step 3 : To balance the number of sodium atoms
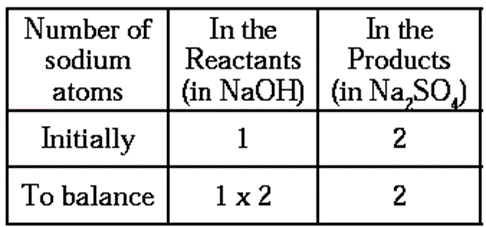
To equalise the number of sodium atoms, we use 2 as the factor of NaOH in the reactants. Now, the partly balanced equation becomes as follows :
2NaOH + H2SO4 ——> Na2SO4+ H2O ….(2)
Check whether the equation (2) is balanced or not.
We find that the equation (2) is not balanced, as the number of oxygen (6 | 5) and hydrogen atoms (4 | 2) are unequal on the two sides.
First balance the hydrogen number as it requires a smaller factor.
Step 4 : Now, balance the number of hydrogen atoms :
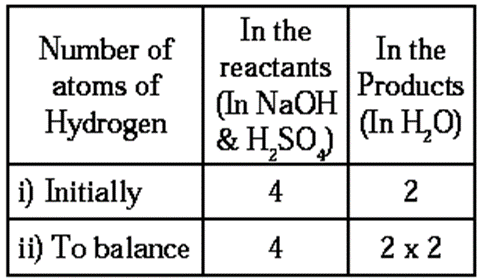
To equalise the number of hydrogen atoms, we use 2 as the factor of H2O in the products. The equation then becomes
2NaOH + H2SO4 ——> Na2SO4+ 2H2O ….(3)
Now, count the atoms of each element on both sides of the equation. The number of atoms on both sides are equal. Hence the balanced equation is
2NaOH + H2SO4 ——> Na2SO4+ 2H2O
In this way, a balanced equation is obtained from an unbalanced equation by applying proper factors to appropriate reactant/product so as to balance the number of each element in steps.
Example II:
Let us take a little more difficult equation, when iron is combined with steam (H2O).
Step 1 : Write the skeletal equation from the given word equation.
The skeletal equation for the above reaction is
Fe + H2O —-> Fe3O4 + H2
Stop 2 : Write the number of atoms of each element in the unbalanced equation on both sides of equations.
| Reactant (LHS) | Product (RHS) | |
| Element | No of atoms | No of atoms |
| Fe | 1 | 3 |
| H | 2 | 2 |
| O | 1 | 4 |
Then, Fe3O4 is selected which contains the maximum of atoms. (It is convenient to start with the molecule or compound that contains the maximum number of atoms.)
It contains 4 oxygen atoms whereas there is only one oxygen atom on the other side, i.e., L.H.S, in H2O.
Step 3 : To balance the number of Oxygen atom
H2O is multiplied by 4 at LHS
Fe + 4H2O -——-> Fe3O4 + H2
| Reactant (LHS) | Product (RHS) | |
| Element | No of atoms | No of atoms |
| Fe | 1 | 3 |
| H | 2 x 4 =8 | 2 |
| O | 1 x 4 = 4 | 4 |
Again examine the effect of step (3). Oxygen is balanced but Fe and H are not yet balanced.
Step 4 : To balance the number of Hydrogen atoms
Multiply H2 by 4 at RHS
Fe + 4H2O -——> Fe3O4 + 4H2
| Reactant (LHS) | Product (RHS) | |
| Element | No of atoms | No of atoms |
| Fe | 1 | 3 |
| H | 8 | 2 x 4 = 8 |
| O | 4 | 4 |
Examine the effect of step (4).
When counting the number of atoms on both sides, it is seen Fe is one on L.H.S. and is 3 on R.H.S.
Step 4 : To balance the number of Fe atoms
Multiply Fe on L.H.S. by 3.
3Fe + 4H2O ———> Fe3O4 + 4H2
| Reactant (LHS) | Product (RHS) | |
| Element | No of atoms | No of atoms |
| Fe | 1 x 3 =3 | 3 |
| H | 8 | 8 |
| O | 4 | 4 |
Now this is a balanced chemical equation.
3Fe + 4H2O ———> Fe3O4 + 4H2
[collapse]
Balancing by Partial Equation Method :
If the reaction is complicated i.e., it involves large number of reactants and products, it is preferred to write the equation in steps (the actual reaction may or may not be taking in these steps). Each step should be a balanced chemical equation.
For example, when copper reacts with conc. nitric acid, products are cupric nitrate, nitrogen dioxide and water.
Cu + HNO3 -—> Cu(NO3)2 + NO2 + H2O
Step-1 :
We can write this reaction in three steps and balance each step separately.
(i) First, HNO3 is decomposed to give nitrogen dioxide (NO2) and water (H2O) and atomic oxygen.
2HNO3 -—> 2NO2 + H2O + O ...(i)
(ii) In the next step, copper is oxidised to copper (II) oxide (CuO)
Cu + O -—> CuO ...(ii)
(iii) Copper oxide so formed then reacts with nitric acid to form copper nitrate (Cu(NO3)2) and water(H2O).
CuO + 2HNO3 -—> Cu(NO3)2 + H2O …(iii)
Step-2 : Multiply Eqns. (i), (ii), (iii) by an integer so that on adding eq. (i), (ii) and (iii), intermediate products cancel out, i.e. product (CuO in this case ) which do not appear in the final reaction.
Now in this case, we find that the integer is 1 therefore aadd equation as it is
Step-3 : Adding (i), (ii) and (iii)
2HNO3 + Cu + O + CuO + 2HNO3 -—> 2NO2 + H2O + O + CuO + Cu(NO3)2 + H2O
Now O & CuO cancelled out from both side
Step-4 : Write the final balanced equation as shown
Cu + 4HNO3 ——> Cu(NO3)2 + 2NO2 + 2H2O
This method is known as Partial Equation Method.
[collapse]
Symbols of Physical States : To make a chemical equation more informative, the physical states of the reactants and products are mentioned along with their chemical formulae.
- The gaseous, liquid, aqueous and solid states of reactants and products are represented by the notations (g), (l), (aq) and (s), respectively.
- The word aqueous (aq) is written if the reactant or product is present as a solution in water.
- Usually physical states are not included in a chemical equation unless it is necessary to specify them.
- Sometimes the reaction conditions, such as temperature, pressure, catalyst, etc., for the reaction are indicated above and/or below the arrow in the equation.
6CO2(aq) +12H2O(l) \(\frac{sunlight}{chlorophyll}\) > C6H12O6(aq) +6O2(aq) +6H2O(l)
[collapse]
Thermochemical equation :. An equation in which information about heat change is included is called a thermochemical equation.
- Most chemical reactions are accompanied by either evolution or absorption of heat. These reactions are known as exothermic and endothermic reactions respectively
- It is very important in such a case to indicate the physical state of the various species involved.
Exothermic reactions: Chemical reactions in which energy is evolved (or given out) are known as exothermic reactions. For example,
C(s) + O2(g) ———> CO2(g) …. ΔH = −3935 kJ
Endothermic reactions: Reactions in which energy is absorbed are called endothermic reactions. For example,
C(graphite) + 2H2(g) ———-> CH4(g) ….. ΔH = +74.25 kJ
Q. Classify the following as exothermic and endothermic reactions (CBSE 2014,1015)
- Photosynthesis
- Respiration
- Burning of natural gas
- Electrolysis of water
- Decomposition of calcium carbonate.
- Burning of magnesium ribbon in air.
| Chemical Reaction | Type |
| Photosynthesis | Endothermic |
| Respiration | Exothermic |
| Burning of natural gas | Exothermic |
| Electrolysis of water | Endothermic |
| Decomposition of calcium carbonate | Endothermic |
| Burning of magnesium ribbon in air | Exothermic |
[collapse]
Ionic equations : An equation which shows only the atoms and ions that actually take part in the reaction is called an ionic equation.
- Ions which remain as such and do not take part in the reaction are not indicated. .
Example : When zinc metal and dilute sulphuric acid are reacted, then; zinc sulphate and hydrogen gas are formed. Now sulphuric acid exists as 2H+ ions and SO42- ions. Similarly, zinc sulphate exists as Zn2+ ions and SO42- ions. So, the above reaction can be written as:

In this equation, we have one sulphate ion, SO42-, on both the sides, which remains as such and does not take part in the reaction. So, cancelling the sulphate ion from both sides, we get the following ionic equation for the above reaction:
Zn + 2H+ ———> Zn2+ + H2
Balancing of ionic equation :
Example : Ionic equation : Al + 2H+ ——> Al3+ + H2
Al + 2H+ ——> Al3+ + H2 is not a balanced equation.
An ionic equation should be balanced with respect to atoms (or ions) as well as charges.
An ionic equation must be balanced in three steps.
Step-1 : First balance the equation with respect to atoms.
This equation is balanced in respect to the number of atoms.
Step-2 : Then balance’ the equation in respect to charges.
There are 2 positive charges (in 2H+) on the left side but We have 3 positive charges (in Al3+) on the right side.
In order to make charges equal on both sides, multiply 2H+ by 3 and Al3+ by 2, so that we get 6 positive charges on both sides.
Al + 6H+ ——-> 2 Al3+ + H2
Step-2 : Then again balance the equation in terms of atoms if it is disturbed
There is one Al atom on the left but 2 Al3+ on the right. Thus, we should multiply Al by 2 on the left.
Again there are 6H+ ions on left but only 2H atoms in H2 on the right side.
To have 6H on right side, we should multiply H2 by 3 on right.
Now, the above equation becomes
2Al + 6H+ ——-> 2Al3+ + 3H2 …..(Balanced equation)
| LHS | RHS | |
| Number of A1 or Al3+ | 2 | 2 |
| Number of H or H+ | 6 | 6 |
| Number of charges | 6+ | 2 x 3 = 6+ |
[collapse]
Types Of Chemical Reactions :
Combination Reaction : The reaction of two or more substances (either elements or compounds) to produce a new substance is called a combination reaction.
Example : Magnesium (Mg) on burning in air combines with oxygen to give magnesium oxide.
2Mg(s) + O2(g) ———> 2MgO(s)
Decomposition Reaction : The reaction in which a compound splits up into two or more simpler substances is called decomposition reaction.
Thermal decomposition : In most of the decomposition reactions heat is used. This is called thermal decomposition. Therefore this reaction is always endothermic.
- Example : Limestone (CaCO3) decomposes to calcium oxide and carbon dioxide on heating.
CaCO3 \(\overset{heat}{\rightarrow}\) CaO + CO2(g)
- Sometimes light, electricity or catalyst is also used.
[collapse]
Electrolytic decomposition: Water decomposes when electricity is passed through it.
2H2O \(\overset{electricity}{\rightarrow}\) 2H2 + O2
In above chemical reaction two molecules of water on electrolysis give 2 molecules of hydrogen gas and one molecule of oxygen gas or the amount of hydrogen gas collected would be double than that of oxygen gas.
[collapse]
Light Decomposition: Silver chloride when exposed to sunlight turns grey. This is due to decomposition of silver chloride into grey silver and chlorine in the presence of light.
This happens due to absorption of light energy. Such reactions are used in photography.
2AgCl(s) \(\overset{heat}{\rightarrow}\) 2Ag(s) + Cl2(g)
white Grey
Silver bromide also behaves in the same way.
2AgBr(s) \(\overset{heat}{\rightarrow}\) 2Ag(s) + Br2( g)
Brown Grey
Example : Zinc displaces copper from copper sulphate solution.
CuSO4(aq) + Zn(s) ———> ZnSO4(aq) + Cu(s)
Zinc sulphate Copper
Similarly, magnesium displaces hydrogen from hydrochloric acid
Mg(s) + 2HCl(aq) -——-—> MgCl2(aq) + H2(g)
[collapse]
Dissociation :
When a substance breaks up into + ve and — ve ions in water, it is called dissociation.
For example, acetic acid in Water dissociates into CH3COO- and H+ ions.
CH3COOH + H2O ⇔ CH3COO- + H3O+
Hydrochloric acid gas in water dissociates to H+ and Cl- ions.
HCl(g) + H2O(l) ——> H+(aq) (H3+O) + Cl-(aq)
Double decomposition or displacement :
The reaction in which two reacting molecules exchange their partner ions in aqueous solution is double decomposition.
Example : The precipitation of AgCl by adding AgNO3 to NaCl solution.
AgNO3(aq) + NaCl(aq) ———> AgCl(s) + NaNO3(aq)
Types of Combination reactions :
There are three types of combination reactions. These are discussed as follows:
(i) Combination between two elements: In these reactions, two elements combine under suitable conditions to form a compound. A few examples of this type of combination reactions are:
(a) Carbon element burns in oxygen to form carbon dioxide
C + O2 \(\overset{combustion}{\rightarrow}\) CO2
(b) Iron and sulphur elements when heated form iron sulphide
Displacement OR substitution reaction :
The reaction in which an atom or a group of atoms in the molecule is displaced by another atom or a group of atoms is called displacement or substitution reaction.
Fe + S\(\overset{heat}{\rightarrow}\) FeS
(ii) Combination between an element and a compound: In these reactions, one of the combining substances is an element whereas the other is a compound. A few examples of this type of combination reactions are:
(a) Carbon monoxide combines with oxygen to form carbon dioxide.
2CO + O2 ———> 2CO2
(b) Sulphur dioxide combines with oxygen upon heating to form sulphur trioxide.
2SO2 + O2 \(\overset{heat}{\rightarrow}\) 2SO3
Oxidation and Reduction :
Oxidation: It is a process
- In which oxygen or any electronegative element is added up or
- Hydrogen or any electropositive element is removed
Examples :
(a) Sulphur burns in air with a blue flame to form sulphur dioxide. Here oxygen is added up to sulphur.
S(s) + O2(g) —--> SO2(g) (Addition of oxygen to sulphur)
(b) Hydrogen sulphide combines with iodine to give hydrogen iodide and sulphur.
H2S + I2 —--> 2HI + S ….(Removal of hydrogen from H2S)
[collapse]
Reduction: It is a process in which
- hydrogen or an electropositive element is added up.
- Oxygen or electronegative element is removed.
Examples :
(a) Hydrogen reacts with chlorine to form hydrogen chloride
Cl2(g) + H2(g) ——> 2HCl(g) ….(Addition of hydrogen to chlorine)
(b) Copper oxide is reduced with hydrogen.
CuO(s) + H2(g) ——> Cu(s) + H2O(l) …..(Removal of oxygen from CuO)
[collapse]
Electronic concept of oxidation and reduction :
Oxidation: An oxidation reaction is one in which electrons are released or lost.
Magnesium atom is oxidised to magnesium ion by loss of electrons.
Mg —> Mg2+ + 2e
Magnesium atom Magnesium ion Electrons
Reduction: A reaction in which electrons are accepted is called a reduction. Oxygen atom is reduced to oxide ion by accepting two electrons.
O + 2e —> O2-
Oxygen atom Electrons Oxide ion
[collapse]
Oxidising agent and Reducing agent :
Oxidising agent: It is that substance which in a reaction
- gives up oxygen or any electronegative element,
- accepts hydrogen or any electropositive element or
- accepts electrons.
Reducing agent: It is that substance which in a reaction
- gives up hydrogen or any electropositive element,
- accepts oxygen or any electronegative element or
- releases electrons.
In the following reaction:
H2S + Cl2 ——> S + 2HCl
Chlorine accepts hydrogen from H2S, thus oxidising it and is called oxidising agent. It also accepts electron.
Or
Hydrogen sulphide is reducing agent as it gives up hydrogen to chlorine. Here H2 releases electron.
In a chemical reaction oxidising agent is reduced and the reducing agent is oxidised.
In the reaction between zinc and copper sulphate solution
Zn + Cu2-SO42- ——> Zn2+SO42- + Cu
Zn reduces Cu2+ to Cu2, itself being oxidised to Zn2+ or Zn acts as a reducing agent.
[collapse]
Redox reaction : A chemical reaction in which one substance is oxidised and the other is reduced is called a redox reaction.
- All oxldatlon-reduction reactions are redox reactions.
- In a chemical reaction, a substance gets oxidised only when another substance is present, which gets reduced.
CuO + H2 \(\underrightarrow{Heat}\) Cu + H2O
Here CuO is losing oxygen, is being reduced. The hydrogen is gaining oxygen, is being oxidised
- It is true that oxidation cannot take place without reduction and vice versa. As electrons do not exist free, electrons lost during oxidation must be gained during reduction. Such reactions are called redox reactions.
Q. Select the oxidising agent and the reducing agent from the following reactions:
(i) H2S + I2 ——> 2HI + S
(ii) CuO + H2 ——> Cu + H20
(iii) Zn + CuSO4 ——> ZnSO4 + Cu.
(i) H2S is reducing agent and I2 is oxidising agent. (ii) CuO is oxidising agent and H2 is reducing agent. (iii) Zn is reducing agent and CuSO4 is oxidising agent.
Q. State some reactions of oxidation that you observe in your everyday life.
Ans. Some examples of oxidation reactions are:
Q. Can a displacement reaction be a redox reaction? Explain with the help of an example. (CBSE 2015)
Consider the following displacement reaction: Zn + CuSO4 ——> ZnSO4 + Cu Writing this in ionic form, we get Zn + Cu2+ ——-> Zn2+ + Cu Here, zinc atom donates two electrons (to copper atom) and forms a zinc ion, Zn2+. By definition, this is an oxidation reaction. Also copper ion, Cu2+ accepts two electrons (given by zinc atom) and forms copper atom, Cu. By definition, this is reduction. SO42- ions remain as such and hence above reaction involves both reduction as well as oxidation processes and is a redox reaction. Thus, a displacement reaction can be a redox reaction.
Have you observed the effects of oxidation Reactions in everyday life? :
Corrosion : Due to the effect of moisture and acids, metals get corroded. This effect is called corrosion.
Effects of corrosion :
Corrosion causes damage to metal articles lik car bodies, bridges, iron railings, Ships and other substances of daily use.
Corrosion and rusting :
Corrosion is seen in all metals, when exposed to air. A layer of metal compound is formed on the surface due to presence of moisture, acids or other gases present in air.
Rusting : Rusting is the process in which iron metal reacts with air and moisture to form brownish powder called rust.
- Shiny iron articles on exposure to moisture get coated with a brownish powder which peels off easily. In this way enormous amount of iron is damaged and lost.
- Rust is a complex compound of iron and it takes several months to form when iron is exposed to air and moisture, therefor it is called slow reaction. This is a combination reaction.
Prevention of rusting:
- The iron articles should be painted.
- The machine parts should be oiled and greased.
- Galvanised iron pipes are used for water supply.
- Iron can be coated with chromium.
[collapse]
Rancidity : Fats and oils in food kept for long time get oxidised and become rancid and taste of food changes and causes infection on eating. This is called rancidity.
Prevention of Rancidity :
- To prevent rancidity antioxidants (which prevent oxidation) are added to food containing fats and oils.
- Rancidity can also be prevented by flushing out oxygen with an inert gas like nitrogen. For example, packets of food items like chips are flushed with nitrogen so that these can be used even after long duration.
- Keeping food in airtight containers also help to slow down oxidation and to preserve food for a longer time.
Click on below link to get PDF from store
PDF : NCERT Class 10-Science Chapter-1-Chemical Reactions and Equations-Notes
PDF : NCERT Class 10-Science Chapter-1-Chemical Reactions and Equations-Text Books
PDF : NCERT Class 10-Science Chapter-1-Chemical Reactions and Equations- Solution
| Main Page : CBSE-NCERT- Class 10th - Science - All chapters notes, solutions, videos, test, pdf.
Next Chapter : Chapter 2 : Acids, Bases and Salts - Online Notes |