Acids,Bases and Salts
NCERT-Class 10-Science-Chapter-2
Notes-Part-2
Topics to be learn : Part-2
|
More about salts :
Formation of salts:
Salts are formed by combination of acids and bases. For example, salt of sodium sulphate,.Na2SO4 is formed by reaction of sulphuric acid with sodium hydroxide.
pH value of salts in aqueous solution :
- Salts of strong acid and a strong base are neutral with pH value of 7.
- Salts of strong acid and weak base are acidic with pH value less than 7.
- Salts of a strong base and weak acid are basic in nature with pH value more than 7
Combining of acids and bases and salts formed :
| Acids | + Bases | Salts formed |
| H2SO4
H2SO4 H2SO4 H2SO4 H2SO4 HCl HNO3 H2CO3 HCl |
KOH
NaOH Ca(OH)2 Mg(OH)2 Cu(OH)2 NaOH NaOH NaOH NH4OH |
Potassium sulphate (K2SO4)
Sodium sulphate (Na2SO4) Calcium sulphate (CaSO4) Magnesium sulphate (MgSO4) Copper sulphate (CuSO4) Sodium chloride (NaCl) Sodium nitrate NaNO3 Sodium carbonate Na2CO3 Ammonium chloride NH4Cl |
[collapse]
Salt Family :
Salts having the same positive or negative radicals are said to belong to a family.
- For example, NaCl and Na2SO4 belong to the family of sodium salts. Similarly, NaCl and KCl belong to the family of chloride salts.
The following salt families can be identified:
- Family of sulphate salts: K2SO4, Na2SO4, CaSO4, MgSO4, CuSO4
- Family of chloride salts: NaCl, NH4Cl
- Family of sodium salts: Na2SO4, NaCl, NaNO3, Na2CO3
Types of salt :
Neutral or normal salt : It does not hydrolyse in water It does not contains extra H+ or OH− ions.
- Example : Sodium chloride (NaCl)
Acidic salt : It hydrolyses in water to give strong acid.
- Example : Sodium hydrogen sulphate (NaHSO4). It hydrolyses in water to give strong acid H2SO4
Basic salt : It hydrolyses in water to give strong base.
- Example : Sodium acetate (NaOOCCH3). It hydrolyses in water to give strong base, NaOH.
[collapse]
Water of crystallization : Water of crystallization is the fixed number of water molecules present in one formula unit of a salt.
For example, chemical formula of hydrated copper sulphate CuSO4.5H2O. Copper sulphate has 5 molecules of water of crystallization. Sodium carbonate (Na2CO3.10H2O) contains ten molecules of water of crystallization.
Examples of hydrated salts :
(i) Ferrous sulphate, FeSO4.6H2O
(ii) Magnesium sulphate, MgSO4.7H2O
(iii) Barium chloride, BaCl2.2H2O
(iv) Calcium sulphate, CaSO4.2H2O
(v) Sodium thiosulphate, Na2S2O3.7H2O
(vi) Copper sulphate, CuSO4.5H2O.
[collapse]
Q. Why does common salt become moist in rainy season?
Ans. Common salt contains small amounts of magnesium chloride (MgCl2) which is deliquescent and is responsible for sodium chloride to become moist in rainy season.
Important uses of sodium chloride (common salt) :
The common salt thus obtained is an important raw material for various materials of daily use,
- It is an essential constituent of our daily life and is used for making food items.
- It is used for the manufacture of soap.
- Mixed with ice, it is used as a freezing mixture.
- It is used as a preservative for meat, fish and pickles.
- It is used for the industrial preparation of a number of compounds like hydrochloric acid, washing soda, caustic soda etc.
[collapse]
Sodium hydroxide :
An important alkali commonly needed for laboratory work is sodium hydroxide.
It can be prepared from sodium chloride by the process of electrolysis. This is called chlor-alkali process.
Electrolysis of aqueous solution of sodium chloride:
- When electricity is passed through an aqueous solution of sodium chloride commonly called brine, it decomposes into chloride and sodium.
- Sodium is collected at the cathode where it reacts with water to form sodium hydroxide.
- Chlorine is formed at the anode and is collected as a gas.
2NaCl \(\overset{Electrolysis}{\rightarrow}\) 2Na + 2Cl
At Cathode: 2Na + 2H2O → 2NaOH(aq) + H2(g)
At Anode: CL + CL → Cl2(g)
The overall reaction is
2NaCl(aq) + 2H2O(l) \(\overset{Electrolysis}{\rightarrow}\) 2NaOH(aq) + Cl2(g) + H2(g)
Note : The one product of chlor-alkali process is sodium hydroxide (NaOH). Dry
NaOH absorbs moisture and turns sticky. It is neutralized when treated with strong acid.
NaOH + HCl → NaCl + H2O
(NaOH) cannot be stored in aluminium vessel as it reacts with aluminium and will corrode it because it gives soluble sodium aluminate.
Products of electrolysis of brine (sodium chloride) and their uses :
Products of electrolysis of brine are:
(i) chlorine gas, (ii) H2 gas and (iii) sodium hydroxide.
Uses of chlorine gas:
- It is used for water treatment by MCD.
- It is used in swimming pools as disinfectant.
- It is used for the manufacture of PVC, CFCs, bleaching powder and pesticides.
Uses of H2 gas:
- It is used as fuel.
- It is used for the manufacture of ammonia.
- It is used for manufacture of ghee, margarine etc.
Uses of NaOH:
- It is used for degreasing metals.
- It is used for the manufacture of soaps and detergents.
- It is used in artificial fibre industry.
[collapse]
Sodium carbonate (Washing soda) :
Nowadays sodium carbonate is manufactured on a large-scale by Solvay ammonia process and involves the following steps:
NH3(g) + H2O(l) + CO2(g) → NH4HCO3(aq) (Ammonium bicarbonate) NH4HCO3(aq) + NaCl(aq) → NH4Cl(aq) + NaHCO3(s) (Sodium bicarbonate) 2NH4Cl + Ca(OH)2 → CaCl2 + 2H2O + ZNH3 2NaHCO3(s) \(\overset{Heat}{\rightarrow}\) Na2CO3(s) + H2O(l) + CO2(g) Ammonium chloride and carbon dioxide are used again and again and so the manufacture of sodium carbonate involves only sodium chloride and limestone as the cheap raw materials. The overall flow chart is given below in Fig. Thus only limestone and sodium chloride are consumed and washing soda can be continuously manufactured.
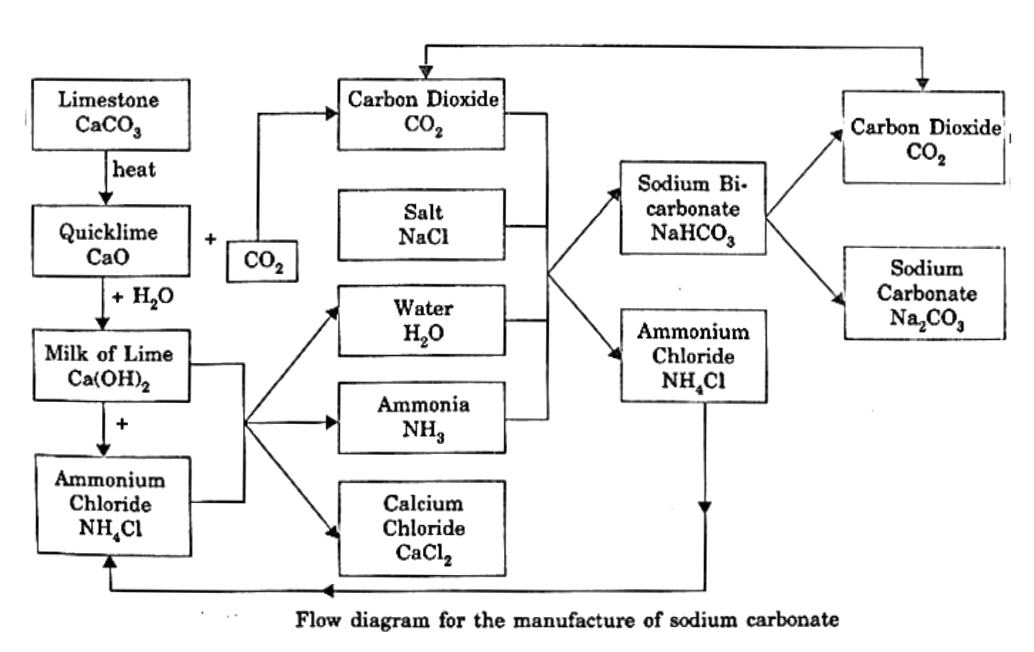
Properties of sodium carbonate: When anhydrous, it melts at 852°C. It dissolves in water with evolution of heat. Its aqueous solution is alkaline due to hydrolysis and its solution turns red litmus blue. Na2CO3(s) + H2O(l) → 2NaOH(aq) + CO2(g) Upon cooling its hot concentrated solution, this gives Na2CO3.10H2O (decahydrate). When this variety is exposed to air, it efflorescences and finally changes into monohydrate Na2CO3.H2O. Upon strong heating, this changes into anhydrous form. It reacts with mineral acids to give carbon dioxide gas. . Na2CO3(s) + 2HCl(aq) → 2NaCl(s) + CO2(g) + H2O(l). Uses of sodium carbonate :
Q. Why Potassium carbonate cannot be made by Solvay’s process using potassium chloride in place of sodium chloride?
Ans. Potassium hydrogen carbonate, KHCO3 is highly soluble in water and, therefore, does not crystallize out as does sodium hydrogen carbonate.
Efflorescence : Crystals of certain substances lose water and turn into a powder For example, Na2CO3.10H2O crystals on being left in the open turn into powder, Na2CO3.H2O. This giving up of water of crystallization by crystals to the atmosphere is termed as efflorescence.
Sodium bicarbonate used in a fire extinguisher :
A soda acid fire extinguisher consists of a metal container filled with a solution of sodium bicarbonate. A glass bottle kept inside the container has sulphuric acid in it. When the knob of the extinguisher is pressed, the bottle breaks. The acid comes in contact with sodium bicarbonate and carbon dioxide is produced.
2NaHCO3 + H2SO4 → Na2SO4 + 2H2O + 2CO2
The carbon dioxide comes out through the nozzle which is directed towards the fire.
Carbon dioxide cuts off the supply of air, thus the fire gets extinguished.

[collapse]
Carbonation : Carbonation is a process in which brine (NaC1) saturated with ammonia is allowed to come in contact with carbon dioxide under pressure to form sodium bicarbonate (NaHCO3 -washing soda).
NH3(g) + H2O(l) + CO2(g) → NH4HCO3(aq)
NH4HCO3(aq)+ NaCl(aq) → NH4Cl(aq) + NaHCO3(s)
Baking powder: It is a mixture of sodium bicarbonate and small amounts of corn starch and hydrogen tartarate. The chemical name of baking powder is sodium hydrogen carbonate.
Preparation: Carbon dioxide is passed through a saturated solution of sodium carbonate.
Sodium bicarbonate, being sparingly soluble in water gets precipitated which is washed and dried in air. Requisite amounts of corn starch and hydrogen tartarate are then mixed,
Na2CO3 + CO2 + H2O → 2NaHCO3(s) (Sodium hydrogen carbonate)
It can also be prepared using sodium chloride as raw material.
NaCl + H2O + CO2 + NH3 → NH4Cl + NaHCO3 (Ammonium chloride+ Sodium hydrogen carbonate)
Properties:
- It is alkaline in nature.
- At 100°C, it decomposes with evolution of CO2 gas.
2NaHCO3 → Na2CO3 + CO2 + H2O.
Uses: It is used
- as a cooking agent.
- in medicines for neutralising acidity in stomach
- in soft drinks.
- in fire extinguishers.
[collapse]
Baking Soda (Sodium bicarbonate) :
Baking soda on mixing with other ingredients gives baking powder.
Baking powder contains baking soda, corn and tartaric acid. NaHCO3 in baking powder on heating gives Na2CO3 and CO2.
2NaHCO3 → Na2CO3 + CO2 + H2O
- Baking soda is used in small amount for making bread and cake. It helps to make these soft and spongy.
- On heating, sodium bicarbonate decomposes to produce carbon dioxide. This causes biscuits and cakes etc. to expand and become light.
- The other constituents act as preservatives.
- Tartaric acid acts as preservative and also reacts with baking soda to give carbon dioxide.
- Baking soda help when we feel pain and irritation in the stomach during indigestion.
- Pain and irritation in the stomach during indigestion is felt when the acidity in the stomach increases. Baking soda is a mild base and neutralises the excess acid.
- Baking soda is a mild base
- The pH of baking soda solution is slightly greater than seven. An aqueous solution of baking soda turns red litmus blue.
Q. For making cake, baking powder is taken. If at home your mother used baking soda instead of baking powder in cake then how will it affect the taste of cake and why?
Ans. For making cake, baking powder is taken. If baking soda is used instead of baking powder in cake then the taste of cake becomes bitter as the medium becomes too basic due to baking soda.
Bleaching powder :
CaOCl2 (calcium oxychloride) is bleaching powder.
Preparation of bleaching powder:
This is prepared by passing chlorine gas over slaked lime for a long time.
Ca(OH)2(s) + Cl2(g) → CaOCl2(s) + H2O(l)
Properties of bleaching powder :
- Bleaching powder is a yellowish powder with smell of chlorine.
- When in atmosphere, it absorbs moisture but is not deliquescent.
- It is soluble in water but a small insoluble portion is left behind due to the lime present in it.
- Action of carbon dioxide: Upon reaction with carbon dioxide (air), it deteriorates giving off chlorine and calcium carbonate,
CaOCl2(s) + CO2(g) → CaCO3(s) + Cl2(g).
- Bleaching powder smells strongly of chlorine because it slowly reacts with carbon dioxide of air to evolve chlorine gas.
- Action of dilute acids : With dilute acids, it liberates the whole of its chlorine,
CaOCl2(s) + H2SO4(aq) → CaSO4(s) + H2O(l) + Cl2(g)
CaOCl2(s) + 2HCl(aq) → CaCl2(s) + H2O(l) + Cl2(g)
- Effect on long standing: Upon long standing, the following reaction takes place,
6CaOCl2(s) → 5CaCl2 + Ca(ClO3)2
- Thus, the quantity of available chlorine decreases.
Important uses of bleaching powder :
- Used for making drinking water free from germs.
- Used in manufacture of chloroform.
- Used for bleaching cotton and wood pulp in textiles etc.
- Used for making wool unshrinkable.
Q. Why are commercial samples of bleaching powder not completely soluble in water.
Ans. Bleaching powder is soluble in water. However, commercial samples of bleaching powder contain slaked lime that does not react with chlorine gas during the manufacture of bleaching powder. The insoluble part of bleaching powder is this white solid, i.e., slaked lime.
Antichlor : Antichlor is a substance used to remove excess of chlorine from a material/cloth. Examples are sodium bisulphite and sodium thiosulphate.
Use :
After the clothes are treated with bleaching powder and acid solutions, some chlorine remains sticking to the fibre which may injure it if not removed quickly after bleaching is over. Chlorine is removed by reaction with sodium bisulphite as given below:
NaHSO3 + Cl2 + H2O → NaHSO4 + 2HCl.
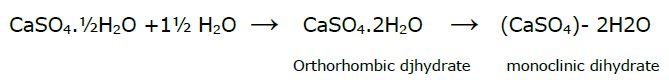
Plaster of Paris : CaSO4.½H2O is known as plaster of Paris. This is prepared by heating gypsum to 120-130°C
2CaSO4.2H2O \(\overset{\tex{Heat 120-130°C}}{\rightarrow}\) (CaSO4)2 H2O + 3H2O(g)
- When Plaster of Paris is heated beyond 120°C, it loses whole of water of crystallisation and anhydrous calcium sulphate is formed. This is called dead burnt plaster.
- Plaster of Paris is written as CaSO4.½H2O, because in this compound 2 calcium sulphate molecules are attached to one water molecule.
Uses of Plaster of Paris:
- It is used in making chalks and fire proof materials.
- Used for making patient plasters used in surgery and for plastering fractured parts of the body.
- Mixed with alum, it is used as a cement in ornamental casting and for making moulds in pottery work.
Properties of Plaster of Paris:
- It is a white powder.
- Setting property: When wetted with water, this forms a solid plastic mass and heat is given out during this process and finally a hard porous mass results within 10—15 minutes. This involves two steps. Firstly, water is absorbed to form orthorhombic dihydrate and change to monoclinic, when the plaster hardens, thus setting takes place.
Q. CaSO4.½H2O is used as plaster of Paris but CaSO4 is used as a drying agent and cannot replace plaster of Paris. Explain.
Ans. Anhydrous calcium sulphate (CaSO4) takes up water readily and forms hydrated salt and so is used as a drying agent, CaSO4 + 2H2O → CaSO4.2H2O CaSO4.½H2O has a particular crystal structure which imparts property of setting and hardening on mixing with water. CaSO4 cannot replace plaster of Paris because on mixing with water it does not acquire the property of setting and hardening.
Click on below link to get PDF from store :
NCERT Class 10-Science Chapter-2-Acids,Bases and Salts-Notes
NCERT Class 10-Science Chapter-2-Acids,Bases and Salts-Exercise Solutions
NCERT Class 10-Science Chapter-2-Acids,Bases and Salts-Text Books
NCERT Class 10-Science Chapter-2-Acids,Bases and Salts-Intext Solution
NCERT Class 10-Science Chapter-2-Acids,Bases and Salts-Exemplar
| Main Page : CBSE-NCERT- Class 10th - Science - All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter 1 : Chemical Reactions and Equations - Online Notes Next Chapter : Chapter 3 : Metals and Non-Metals - Online Notes |