Heat
Based on Class 10-Science & Technology Part-1-Chapter-5- Maharashtra Board-Solutions, Videos, PDF
Solution
Question 1:
Fill in the blanks and rewrite the sentence.
1. The amount of water vapour in air is determined in terms of its ........... .
2. If objects of equal masses are given equal heat, their final temperature will be different. This is due to difference in their ...............
3. During transformation of liquid phase to solid phase, the latent heat is ...........
1. The amount of water vapour in air is determined in terms of its absolute humidity. 2. If objects of equal masses are given equal heat, their final temperature will be different. This is due to difference in their different specific heat capacity. 3. During transformation of liquid phase to solid phase, the latent heat is latent heat of fusion.
Question 2:
Observe the following graph. Considering the change in volume of water as its temperature is raised from 0°C, discuss the difference in the behaviour of water and other substances. What is this behaviour of water called?
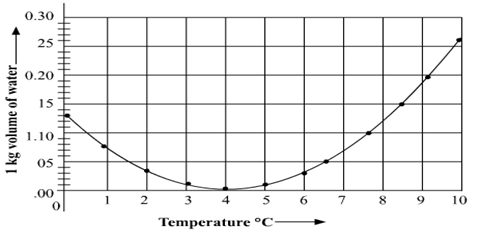
Question 3:
What is meant by specific heat capacity? How will you prove experimentally that different substances have different specific heat capacities?
Specific heat capacity of a body is the amount of heat energy required to raise the temperature of unit mass of that body through 1oC (or 1 K). It is given as ΔT=Rise in temperature m=Mass of the body Experiment to prove different substances have different specific heat capacities:
\(s=\frac{∆Q}{∆T}×m\)
where,
ΔQ=Amount of heat energy supplied
Question 4:
While deciding the unit for heat, which temperature interval is chosen? Why?
The amount of heat energy required to raise the temperature of a unit mass of an object by 1°C is called the specific heat capacity or simply specific heat of the object. While deciding the unit for heat (which is calorie), the temperature interval chosen is 14.5oC-15.5oC. It is, therefore, essential to define a specific temperature range while defining the unit of heat. The amount of heat necessary to raise the temperature of 1 kg of water by 1°C from 14.5 °C to : 15.5 °C is called one kilocalorie.
If we heat 1 kg of water by 1°C in different temperature range than 14.5°C to 15.5°C, the amount of heat required will be slightly different.
The amount of heat necessary to raise the temperature of 1 g of water by 1°C from 14.5 °C to 15.5 °C is called one calorie.
Question 5:
Explain the following temperature vs time graph.
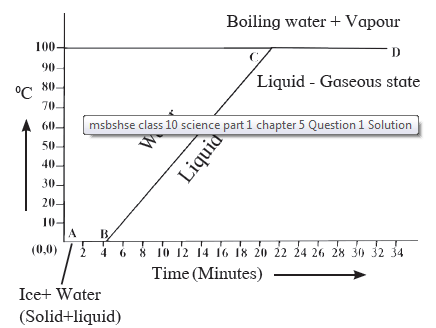
Question 6:
Explain the following:
1. What is the role of anomalous behaviour of water in preserving aquatic life in regions of cold climate?
2. How can you relate the formation of water droplets on the outer surface of a bottle taken out of refrigerator with formation of dew?
3. In cold regions in winter, the rocks crack due to anomolous expansion of water.
Water has the property of expanding below 4oC, is called the anomalous behaviour of water. Thus, in cold regions when the temperature falls below 4oC, the water content present in rocks expands. Due to this expansion of water or increase in volume of water, the rocks cracks.
Question 7:
Answer the following:
1. What is meant by latent heat? How will the state of matter transform if latent heat is given off?
2 Which principle is used to measure the specific heat capacity of a substance?
If a system of two objects is isolated from the environment by keeping it inside a heat resistant box, then no energy can leave the box or enter the box. In this situation, heat energy lost by the hot object = heat energy gained by the cold object. In due course, the two objects attain the same temperature. This is called Principal of Heat Exchange Principle of heat exchange is used in the calorimetry method to determine the specific heat capacity of a substance.
3. Explain the role of latent heat in the change of state of a substances? According to the kinetic model, the total energy of a molecule is the sum of kinetic energy due to its motion (which depends on temperature) and its potential energy (which depends on the force of attraction between the molecules and the separation between them. When a solid is heated, initially, its temperature increases. Here, the heat absorbed by substance is used in increasing the kinetic energy of the particles (atomic, molecules, etc.) of the substance as well as for doing work against the forces of attraction between them. As the heating is continued, at a certain temperature (melting point), solid is converted into liquid. In this case, the temperature remains constant and the heat absorbed is used for weakening the bonds and conversion into liquid phase (liquid state). This heat is called the latent heat of fusion. When a liquid is converted into the gaseous state, at the boiling point, the heat absorbed is used for breaking the bonds between the atoms or molecules. This heat is called the latent heat of vaporization. Some solids, under certain conditions, are directly transformed into the gaseous phase. Here the heat is absorbed but the temperature remains constant. The absorbed heat is used for breaking the bonds between atoms or molecules. This heat is called the latent heat of sublimation. In general, latent heat is the heat absorbed or given out by a substance during a change of state at constant temperature. In transformations from liquid to solid, gas to liquid and gas to solid, latent heat is given out by the substance.
4. On what basis and how will you determine whether air is saturated with vapour or not?
Question 8:
Read the following paragraph and answer the questions.
If heat is exchanged between a hot and cold object, the temperature of the cold object goes on increasing due to gain of energy and the temperature of the hot object goes on decreasing due to loss of energy.
The change in temperature continues till the temperatures of both the objects attain the same value. In this process, the cold object gains heat energy and the hot object loses heat energy. If the system of both the objects is isolated from the environment by keeping it inside a heat resistant box (meaning that the energy exchange takes place between the two objects only), then no energy can flow from inside the box or come into the box.
i. Heat is transferred from where to where?
ii. Which principle do we learn about from this process?
iii. How will you state the principle briefly?
iv. Which property of the substance is measured using this principle?
(i) Heat is transferred from the object at higher temperature to the object at lower temperature. (ii) We learn the principle of heat exchange from this process. (iii) Principle of heat exchange states that If a system of two objects is isolated from the environment by keeping it inside a heat resistant box, then no energy can leave the box or enter the box. In this situation, heat energy lost by the hot object = heat energy gained by the cold object.In due course, the two objects attain the same temperature. (iv) Specific heat of an object can be measured using this principle.
Question 9:
Solve the following problems:
1. Equal heat is given to two objects A and B of mass 1 g. Temperature of A increases by 3°C and B by 5°C. Which object has more specific heat? And by what factor?
Given - m1=100g, Δ T1=3 °C, Δ T2=5 °C, Q=Same Specific heat capacity of a body is given as Q=mc1 Δ T1= mc2 ΔT2 \(∴\frac{c_1}{c_2} = \frac{ΔT_2}{ΔT_1}=\frac{5°C}{3°C}=\frac{5}{3}\) Thus c1>c2 Thus, specific heat capacity of body A is more than body B and by a factor of 5/3.
2.Liquid ammonia is used in ice factory for making ice from water. If water at 20°C is to be converted into 2 kg ice at 0°C, how many grams of ammonia are to be evaporated? (Given: The latent heat of vaporization of ammonia= 341 cal/g)
Given m1=2 kg, ΔT1=20 °C-0°C = 20 °C, c1= 1 kcal/kg.°C, L1 (ice)=80 kcal/kg, L2 (vaporisation of ammonia)=341 cal/g = 341 kcal/kg m2=? Q1 (heat lost by water) =m1c1 Δ T1 + m1L1 = 2kg × 1 kcal/kg.°C × 20°C + 2kg × 80 kcal/kg = 40+160=200 kcal Q2 (heat absorbed by ammonia) = m2L2 = m2 × 341 kcal/kg According to principle of heat exchange Q1=Q2 ∴ 200 kcal = m2 × 341 kcal/kg ∴ m2 = 200/341 kg =0.5865 kg = 586.5 g
3. A thermally insulated pot has 150 g ice at temperature 0°C. How much steam of 100°C has to be mixed to it, so that water of temperature 50°C will be obtained? (Given : latent heat of melting of ice = 80 cal/g, latent heat of vaporization of water = 540 cal/g, specific heat of water = 1 cal/g °C)
Given : m1 = 150 g, ΔT1=50°C-0°C=50 °C, Q2 (heat absorbed by water formed on melting of ice) = m1 Cw ΔT1 = 150 g x 1 cal/g.°C x 50°C = 7500 cal Q3 (heat given out by steam) = m2 L2 = m2 x 540 cal/g Q4 (heat given out by water formed on condensation of steam) = m2 Cw ΔT2 = m2 x 1 cal/g.°C x 50°C = m2 x 50 cal/g According to the principle of heat exchange,
Cw=1 cal/g.°C, L1 = 80 cal/g, L2 = 540 cal/g,
ΔT2 = 100°C-50°C = 50°C, m2 = ?
Q1 (heat absorbed by ice)= m1L1 = 150 g x 80 cal/g = 12000 cal
Q1+Q2= Q3+Q4
∴ 12000 cal +7500 cal = m2 x 540 cal/g + m2 x 50 cal/g
∴ 19500 cal = m2 (540 +50) cal/g
∴ m2 = 19500/590 = 33.05 g
33.05 g of steam is to be mixed.
4.A calorimeter has mass 100 g and specific heat 0.1 kcal/ kg °C. It contains 250 gm of liquid at 30°C having specific heat of 0.4 kcal/kg °C. If we drop a piece of ice of mass 10 g at 0°C, What will be the temperature of the mixture?
Given: m1 = 100 g, c1 = 0.1 kcal/kg.°C=0.1 cal/g.°C, T1 = 30°C m2 = 250 g, C2=0.4 kcal/kg.°C = 0.4 cal/g.°C, T2 = 30°C m3 = 10 g, T3=0°C, L = 80 cal/g, T = ? c (water)=1 cal/g.°C Q1 (heat lost by calorimeter)=m1 c1 (T1-T) T1>T3 , T2>T3, T>T3 According to the principle of heat exchange, Q1+Q2=Q3+Q4 ∴ m1 c1(T1-T)+ m2c2(T2-T)= m3L + m3c(T-0°C) ∴ m1 c1T1 - m1c1T+ m2c2T2- m2c2T= m3L + m3c (T-0°C) ∴ m1 c1T1 + m2c2T2 = m3L + m3c (T-0°C) + m1c1T + m2c2T ∴ m1 c1T1 + m2c2T2 = m3L +(m3c + m1c1 + m2c2)T ∴ 100 x 0.1 x 30 + 250 x 0.4 x 30 = 10 x 80 +(100 x 0.1+250 x 0.4+10 x 1) T ∴ (10 + 100 +10) T =(300 + 3000 - 800)°C ∴ 120 T = 2500°C ∴ T= 2500/120 °C =20.83°C 20.83°C is the temperature of the mixture.
Q2 (heat lost by liquid) = m2c2 (T2-T)
Q3 (heat absorbed by ice) = m3L
Q4 (heat absorbed by water formed on melting of ice) = m3c (T- T3) = m3c (T-0°C)
Click on below link to get PDF from store
MSBSHSE-Class 10-Science & Technology-1-Chapter-5-Heat-Notes
Useful links :
| Main Page : - Maharashtra Board Class 10 Science & Technology Part-1,Part-2 - All chapters notes, solutions, videos, test, pdf.
Books : MSBSHSE -Class 10th Science Text Books – Chapter wise PDF for download Videos : Class 10th Videos of all chapters Previous Chapter : Chapter 4: Effects of Electric Current - Online Solutions Next Chapter :Chapter 6. Refraction of light- Online Solutions |

Nice
Thankyou.Thankyou 👍👊🙊🙉🙈🌹