Structure of Atoms
Based on NCERT Class 11th Chemistry Chapter 2
Theory
Unit II: Structure of Atom
Learn :
• Discovery of electron, proton and neutron and their characteristics;
• Thomson, Rutherford and Bohr atomic models;
• Quantum mechanical model of atom;
• Electromagnetic radiation and Planck’s quantum theory;
• Photoelectric effect, atomic spectra;
• de Broglie relation and Heisenberg uncertainty principle;
• Atomic orbital in terms of quantum numbers;
• Aufbau principle, Pauli exclusion principle and Hund’s rule of maximum multiplicity
• Electronic configurations of atoms.
Atom : Atom is the smallest indivisible particle of the matter. Atom is made of electron, proton and neutrons.
In the early 1800s, the English Chemist JOHN DALTON led to the acceptance of the idea of atoms as neutral , tiny & indivisible particles .
The main postulates of his theory:
- Elements are made of tiny particles called atoms.
- All atoms of a given element are identical.
- The atoms of a given element are different from those of any other element.
- Atoms of one element can combine with atoms of other elements to form compounds.
- A given compound always has the same relative numbers and types of atoms.
- Atoms are indivisible in chemical processes. That is, atoms are not created or destroyed in chemical reactions.
- A chemical reaction simply changes the way the atoms are grouped together.
Drawbacks / Limitations :
- An atom can be further subdivided into protons, neutrons and electrons. However an atom is the smallest particle, which takes part in chemical reactions.
- Atoms of some elements vary in their mass and density. Chlorine has two isotopes having mass numbers 35 a.m.u and 37 a.m.u. Argon and Calcium atoms have the same atomic mass of 40 & are called isobars.
- Simple whole number ratio is not seen in complex organic compounds like sugar C12H22O11.
- The theory completely fails to explain the existence of allotropes
Merits of Dalton's atomic theory :
It has enabled us to explain the laws of chemical combination.
Dalton was the first person to recognize a workable distinction between the ultimate particle of an element (atom) and that of a compound (molecule).
a) ELECTRONS : The first atomic particle to be discovered was the electron.
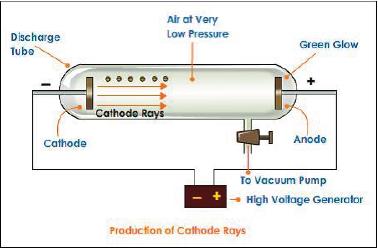
The electron was discovered by J J Thomson during the studies of the passage of electricity through gases at extremely low pressures. Under ordinary conditions , gases are poor conductors of electricity. However, when a high voltage is applied to them at very low pressures, the gases become conductors and electricity begins to flow in the form of rays. These rays are called cathode rays.

Cathode ray discharge tube experiment: A cathode ray discharge tube made of glass is taken with two electrodes. At very low pressure and high voltage,current starts flowing through a stream of particles moving in the tube from cathode to anode. These rays were called cathode rays.
When a perforated anode was taken, the cathode rays struck the other end of the glass tube at the fluorescent coating and a bright spot on the coating was developed
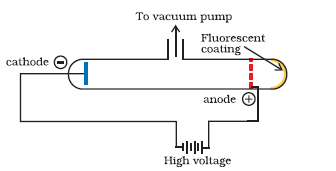
A cathode ray discharge tube with perforated anode
Results:
a. Cathode rays consist of negatively charged electrons.
b. Cathode rays themselves are not visible but their behavior can be observed with help of fluorescent or phosphorescent materials.
c. In absence of electrical or magnetic field cathode rays travel in straight lines
d. In presence of electrical or magnetic field, behavior of cathode rays is similar to that shown by electrons
e. The characteristics of the cathode rays do not depend upon the material of the electrodes and the nature of the gas present in the cathode ray tube.
Characteristics of an electron :
Mass : The mass of an electron is 1/1837 of the mass of a hydrogen atom. Since the mass of hydrogen atom is 1 amu., the relative mass of an electron is 1/1840 amu. The absolute mass is 9 x 10-28gram = 9.1094 x 10-31kg
Charge : An electron is found to carry 1.6 x 10-19 coulomb of negative charge. Since this is the
smallest negative charge carried by any particle, it is taken as unit negative charge.
Charge to mass ratio of an electron was determined by Thomson. The charge to mass ratio of an electron as 1.758820 x 1011 C kg-1
Charge on an electron was determined by R A Millikan by using an oil drop experiment. The value of the charge on an electron is -1.6 x 10-19C.
The mass on an electron was determined by combining the results of Thomson’s experiment and Millikan’s oil drop experiment.
b) PROTON : The smallest and lightest positive ion was obtained from hydrogen and was called
proton.
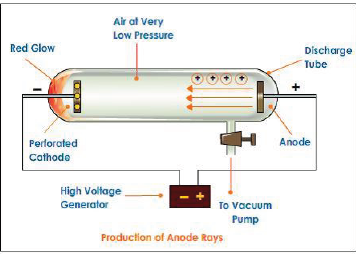
Discovery of protons and canal rays: Modified cathode ray tube experiment was carried out which led to the discovery of protons.
Atom is electrically neutral. The emission of negatively charged electrons from the atoms of the gases in the discharge tube suggest that some positively charged particles must also be present in them.
In 1886, E.Goldstein discovered that in addition to cathode rays, a new kind of rays streaming behind the cathode in discharge tube .These rays traveled in opposite direction to the cathode rays. These rays consist of positively charged particles and are named anode rays.
Mass : Proton is 1837 times heavier than an electron.
Mass of proton is 1.0072766 a.m.u. or 1.6726 x 10-27 kg which is same as that of a hydrogen atom.
Charge : Charge of proton is 1.6022 x 10-19 coulomb.
Since this is the smallest positive charge carried by a particle, so this is taken as the unit of positive charge and we say that the relative charge of a proton is + 1.
Characteristics of positively charged particles:
a. Charge to mass ratio of particles depends on gas from which these originate
b. The positively charged particles depend upon the nature of gas present in the cathode ray discharge tube
c. Some of the positively charged particles carry a multiple of fundamental of electrical charge.
d. Behavior of positively charged particles in electrical or magnetic field is opposite to that observed for cathode rays
Neutrons: Neutrons were discovered by James Chadwick by bombarding a thin sheet of beryllium by α- particles. They are electrically neutral particles having a mass slightly greater than that of the protons.
Atomic number (Z) : the number of protons present in the nucleus (Moseley 1913).
Mass Number (A) :Sum of the number of protons and neutrons present in the nucleus.
ATOMIC MODELS : The major problems before the scientists after the discovery of sub-atomic particles were:
• to account for the stability of atom,
• to compare the behaviour of elements in terms of both physical and chemical properties,
• to explain the formation of different kinds of molecules by the combination of different atoms and,
• to understand the origin and nature of the characteristics of electromagnetic radiation absorbed or emitted by atoms.
Different atomic models were proposed to explain the distributions of these charged particles in an atom. Although some of these models were not able to explain the stability of atoms,
Two of these models, one proposed by J.J. Thomson and the other proposed by Ernest Rutherford are discussed below.
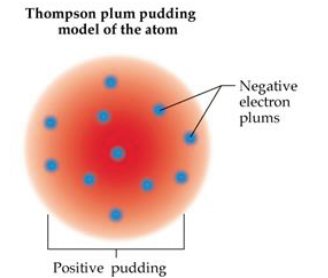
Thomson model of an atom: This model proposed that atom is considered as a uniform positively charged sphere and electrons are embedded in it.
An important feature of Thomson model of an atom was that mass of atom is considered to be evenly spread over the atom.
Thomson model of atom is also called as Plum pudding, raisin pudding or watermelon model
Thomson model of atom was discarded because it could not explain certain experimental results like the scattering of α- particles by thin metal foils.
For Thomson’s atomic theory model he was awarded noble prize in 1906.
Observations from α- particles scattering experiment by Rutherford:
a. Most of the α- particles passed through gold foil un deflected
b. A small fraction of α- particles got deflected through small angles
c. Very few α- particles did not pass through foil but suffered large deflection nearly 180o
Conclusions Rutherford drew from α- particles scattering experiment:
a. Since most of the α- particles passed through foil undeflected, it means most of the space in atom is empty
b. Since some of the α- particles are deflected to certain angles, it means that there is positively mass present in atom
c. Since only some of the α-particles suffered large deflections, the positively charged mass must be occupying very small space.
d. Strong deflections or even bouncing back of α-particles from metal foil were due to direct collision with positively charged mass in atom
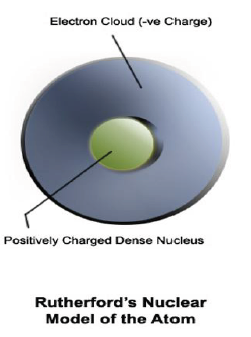
Rutherford’s model of atom: This model explained that atom consists of nucleus which is concentrated in a very small volume. The nucleus comprises of protons and neutrons. The electrons revolve around the nucleus in fixed orbits. Electrons and nucleus are held together by electrostatic forces of attraction.
Drawbacks of Rutherford’s model of atom:
a. According to Rutherford’s model of atom, electrons which are negatively charged particles revolve around the nucleus in fixed orbits.
b. Thus the electrons undergo acceleration, according to electromagnetic theory of Maxwell, a charged particle undergoing acceleration should emit electromagnetic radiation. Thus, an electron in an orbit should emit radiation. Thus, the orbit should shrink. But this does not happen.
c. The model does not give any information about how electrons are distributed around nucleus and what are energies of these electrons.
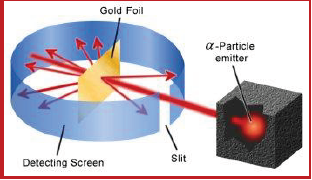
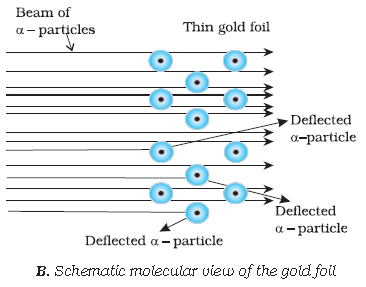
Schematic view of Rutherford’s scattering experiment. When a beam of alpha (a) particles is “shot” at a thin
gold foil, most of them pass through without much effect. Some, however, are deflected.
Isotopes: These are the atoms of the same element having the same atomic number but different mass number.e g 1H1,1H2,1H3
Isobars: Isobars are the atoms of different elements having the same mass number but different atomic number.e g 18Ar40 , 20Ca40
Isotones:- These are atoms of elements which have same number
of Neutrons.
\(_6^{14}C\), \(_7^{15}N\), \(_8^{16}O\)
Isoelectronic species: These are those species which have the same number of electrons.
Electromagnetic radiations: The radiations which are associated with electrical and magnetic fields are called electromagnetic radiations.
When an electrically charged particle moves under acceleration, alternating electrical and magnetic fields are produced and transmitted. These fields are transmitted in the form of waves. These waves are called electromagnetic waves or electromagnetic radiations.
Properties of electromagnetic radiations:
a. Oscillating electric and magnetic field are produced by oscillating charged particles. These fields are perpendicular to each other and both are perpendicular to the direction of propagation of the wave.
b. They do not need a medium to travel. That means they can even travel in vacuum.
Characteristics of electromagnetic radiations:
a. Wavelength: It may be defined as the distance between two neighboring crests or troughs of wave as shown. It is denoted by λ.
b. Frequency (ν): It may be defined as the number of waves which pass through a particular point in one second.
c. Velocity (v): It is defined as the distance traveled by a wave in one second. In vacuum all types of electromagnetic radiations travel with the same velocity. Its value is 3 X108m sec-1. It is denoted by v
d. Wave number: Wave number is defined as the number of wavelengths per unit length.
The frequency (ν ), wavelength (λ) and velocity of light (c) are related by the equation
Velocity = frequency x wavelength
c = νλ
Planck's Quantum Theory: The radiant energy is emitted or absorbed not continuously but discontinuously in the form of small discrete packets of energy called ‘quantum’. In case of light , the quantum of energy is called a ‘photon’
- The energy of each quantum is directly proportional to the frequency of the radiation, i.e. E ∝ υ or E= hυ where h= Planck’s constant = 6.626 x 10-27 Js
- Energy is always emitted or absorbed as integral multiple of this
Black body: An ideal body, which emits and absorbs all frequencies, is called a black body. The radiation emitted by such a body is called black body radiation.
Photoelectric effect: The phenomenon of ejection of electrons from the surface of metal when light of suitable frequency strikes it is called photoelectric effect.
The ejected electrons are called photo electrons.
Experimental results observed for the experiment of Photoelectric effect
- When beam of light falls on a metal surface electrons are ejected immediately.
- Number of electrons ejected is proportional to intensity or brightness of light
Threshold frequency (vo): For each metal there is a characteristic minimum frequency below which photoelectric effect is not observed. This is called threshold frequency.
- If frequency of light is less than the threshold frequency there is no ejection of electrons no matter how long it falls on surface or how high is its intensity.
Photoelectric work function (Wo): The minimum energy required to eject electrons is called photoelectric work function. Wo= hvo
Energy of the ejected electrons : \(h(v-v_o)\) = \(\frac{1}{2}\) \( mv^2\)
Dual behavior of electromagnetic radiation- The light possesses both particle and wave like properties, i.e., light has dual behavior . whenever radiation interacts with matter, it displays particle like properties.(Black body radiation and photoelectric effect) Wave like properties are exhibited when it propagates(interference an diffraction)
When a white light is passed through a prism, it splits into a series of colored bands known as spectrum.
Spectrum is of two types: continuous and line spectrum
a. The spectrum which consists of all the wavelengths is called continuous spectrum.
b. A spectrum in which only specific wavelengths are present is known as a line spectrum. It has bright lines with dark spaces between them.
Electromagnetic spectrum is a continuous spectrum. It consists of a range of electromagnetic radiations arranged in the order of increasing wavelengths or decreasing frequencies. It extends from radio waves to gamma rays.
Spectrum is also classified as emission and line spectrum.
- Emission spectrum: The spectrum of radiation emitted by a substance that has absorbed energy is called an emission spectrum.
- Absorption spectrum is the spectrum obtained when radiation is passed through a sample of material. The sample absorbs radiation of certain wavelengths. The wavelengths which are absorbed are missing
and come as dark lines.
The study of emission or absorption spectra is referred as spectroscopy.
Spectral Lines for atomic hydrogen :
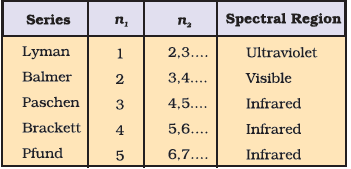
Rydberg equation :
\(\bar v\) = 109677 \(\left(\frac {1}{{n_1}^2} - \frac {1}{{n_2}^2}\right)\)
R = Rydberg’s constant = 109677 cm-1
Bohr’s model for hydrogen atom:
a. An electron in the hydrogen atom can move around the nucleus in a circular path of fixed radius and energy. These paths are called orbits or energy levels. These orbits are arranged concentrically around the nucleus.
b. As long as an electron remains in a particular orbit, it does not lose or gain energy and its energy remains constant.
c. When transition occurs between two stationary states that differ in energy, the frequency of the radiation absorbed or emitted can be calculated.
v = \(\frac{ΔE}{h}\) = \(\frac{E_1 - E_2}{h}\)
An electron can move only in those orbits for which its angular momentum is an integral multiple of h/2π
me v r = n.\(\frac{h}{2π}\) n=1,2,3....
The radius of the nth orbit is given by rn =52.9 pm × \(\frac{n^2}{Z}\)
En = - RH ( \(\frac{1}{n^2}\) )
Limitations of Bohr’s model of atom:
a. Bohr’s model failed to account for the finer details of the hydrogen spectrum.
b. Bohr’s model was also unable to explain spectrum of atoms containing more than one electron.
Dual behavior of matter: de Broglie proposed that matter exhibits dual behavior i.e. matter shows both particle and wave nature.
de Broglie’s relation is : λ = \(\frac{h}{mv}\) = \(\frac{h}{p}\)
Heisenberg’s uncertainty principle: It states that it is impossible to determine simultaneously, the exact position and exact momentum (or velocity) of an electron.The product of their uncertainties is always equal to or greater than h/4π.
Δx × Δp ≥ h/4π
Where Δx = uncertainty in position
Δp = uncertainty in momentum
Heisenberg’s uncertainty principle rules out the existence of definite paths or trajectories of electrons and other similar particles
Failure of Bohr’s model:
a. It ignores the dual behavior of matter.
b. It contradicts Heisenberg’s uncertainty principle.
Classical mechanics is based on Newton’s laws of motion. It successfully describes the motion of macroscopic particles but fails in the case of microscopic particles.
Reason: Classical mechanics ignores the concept of dual behavior of matter especially for sub-atomic particles and the Heisenberg’s uncertainty principle.
Quantum mechanics is a theoretical science that deals with the study of the motions of the microscopic objects that have both observable wave like and particle like properties.
Quantum mechanics is based on a fundamental equation which is called Schrodinger equation.
Schrodinger’s equation: For a system (such as an atom or a molecule whose energy does not change with time) the Schrödinger equation is written as: \(\hat H\) ψ = E ψ
\(\hat H\) is the Hamilton Operator
E is the total energy of the system
ψ represents the wave function which is the amplitude of the electron wave
When Schrödinger equation is solved for hydrogen atom, the solution gives the possible energy levels the electron can occupy and the corresponding wave function(s) of the electron associated with each energy level.
Out of the possible values, only certain solutions are permitted. Each permitted solution is highly significant as it corresponds to a definite energy state. Thus, we can say that energy is quantized.
ψ gives us the amplitude of wave. The value of ψ has no physical significance.
Ψ2 gives us the region in which the probability of finding an electron is maximum. It is called probability density.
Orbital: The region of space around the nucleus where the probability of finding an electron is maximum is called an orbital.
Quantum numbers: There are a set of four quantum numbers which specify the energy, size, shape and orientation of an orbital. To specify an orbital only three quantum numbers are required while to specify an electron all four quantum numbers are required.
Principal quantum number (n):It identifies shell, determines sizes and energy of orbitals
| N | 1 | 2 | 3 | 4 |
| Shell no | K | L | M | N |
| Total number of orbitals in a shell = n2 | 1 | 4 | 9 | 16 |
| Maximum numbers of electron = Zn2 | 2 | 8 | 18 | 32 |
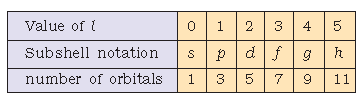
Azimuthal quantum number (l): Azimuthal quantum number. ‘l’ is also known as orbital angular momentum or subsidiary quantum number. l.
It identifies sub-shell, determines the shape of orbitals, energy of orbitals in multi-electron atoms along with principal quantum number and orbital angular momentum, i.e., \(\sqrt {l(l+1) \frac{h}{2π}} \)
The number of orbitals in a subshell = 2l + 1. For a given value of n, it can have n values ranging from 0 to n-1.
Total number of subshells in a particular shell is equal to the value of n.
Magnetic quantum number or Magnetic orbital quantum number (ml): It gives information about the spatial orientation of the orbital with respect to standard set of co-ordinate axis.
For any sub-shell (defined by ‘l’ value) 2l+1 values of ml are possible.
For each value of l, ml = – l, – (l –1), – (l–2)... 0,1... (l – 2), (l–1), l
Electron spin quantum number (ms): It refers to orientation of the spin of the electron. It can have two values +1/2 and -1/2. +1/2 identifies the clock wise spin and -1/2 identifies the anti- clockwise spin.
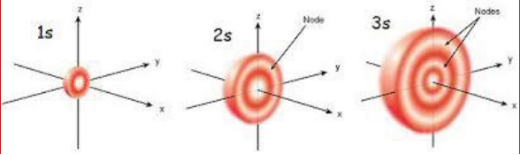
The region where this probability density function reduces to zero is called nodal surfaces or simply nodes.
Radial nodes: Radial nodes occur when the probability density of wave function for the electron is zero on a spherical surface of a particular radius.
Number of radial nodes = n – l – 1
Angular nodes: Angular nodes occur when the probability density wave function for the electron is zero along the directions specified by a particular angle.
Number of angular nodes = l Total number of nodes = n – 1
Degenerate orbitals: Orbitals having the same energy are called degenerate orbitals.
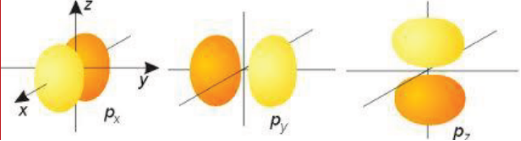
Shape of p and d-orbitals
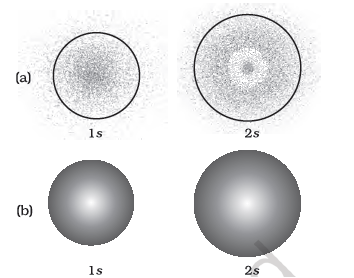
(a) Probability density plots of 1s and 2s atomic orbitals. The density of the dots represents the probability density of finding the electron in that region. (b) Boundary surface diagram for 1s and 2s orbitals.
 |
| s orbitals |
 |
| p orbitals |
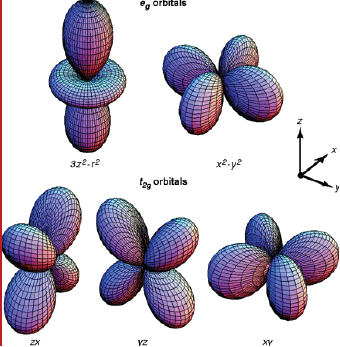 |
| d orbitals |
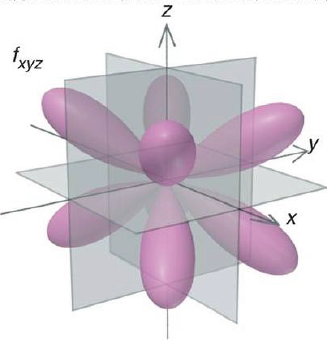 |
| f orbitals |
Shielding effect or screening effect: Due to the presence of electrons in the inner shells, the electron in the outer shell will not experience the full positive charge on the nucleus.
So, due to the screening effect, the net positive charge experienced by the electron from the nucleus is lowered and is known as effective nuclear charge. Effective nuclear charge experienced by the orbital decreases with increase of azimuthal quantum number (l).
Aufbau Principle: In the ground state of the atoms, the orbitals are filled in order of their increasing energies.
n+l rule-Orbitals with lower value of (n+l) have lower energy. If two orbitals have the same value of (n+l) then orbital with lower value of n will have lower energy.
The order in which the orbitals are filled is as follows:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 4f, 5d, 6p, 7s...
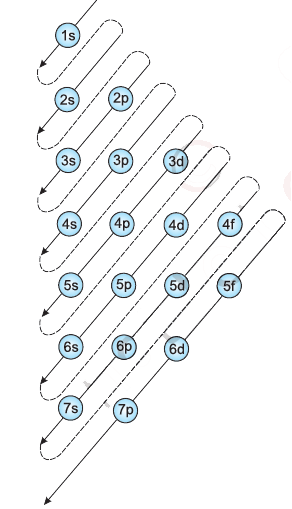
Pauli Exclusion Principle: No two electrons in an atom can have the same set of four quantum numbers. Only two electrons may exist in the same orbital and these electrons must have opposite spin.
Hund’s rule of maximum multiplicity: Pairing of electrons in the orbitals belonging to the same sub shell (p, d or f) does not take place until each orbital belonging to that subshell has got one electron each i.e., it is singly occupied.
Electronic configuration of atoms: Arrangement of electrons in different orbitals of an atom. The electronic configuration of different atoms can be represented in two ways.
a. sapbdc. .... notation.
b. Orbital diagram:, each orbital of the sub-shell is represented by a box and the electron is represented by an arrow (↑) a positive spin or an arrow (↓) a negative spin.
Stability of completely filled and half filled sub-shells:
a. Symmetrical distribution of electrons- the completely filled or half filled sub-shells have symmetrical distribution of electrons in them and are more stable.
b. Exchange energy-The two or more electrons with the same spin present in the degenerate orbitals of a sub-shell can exchange their position and the energy released due to this exchange is called exchange energy. The number of exchanges is maximum when the sub-shell is either half filled or completely filled. As a result the exchange energy is maximum and so is the stability.