Matter in our Surroundings
NCERT Class 9- Science - Chapter 1-Notes-Solution-Video-PDF
NCERT-Class 9-Science-Chapter-1-Matter in our Surroundings-Notes
Topics to be learn :
- Definition of matter; solid, liquid and gas;
- Characteristics - shape, volume, density;
- Change of state-melting (absorption of heat), freezing, evaporation,
[responsivevoice voice="UK English Female" rate="0.8" pitch="0.8" buttontext="Listen to this"]
What is Matter?
Matter is everything in this universe that occupies space and has mass.
Air, water, stones, sand, clouds, pencils, books – Everything is made up of matter.
Constituents of Matter:
Early Indian philosophers classified matter in the form of five basic elements – the “Panchtatava – Air, Water, Earth, Sky, and Fire. According to them everything, living or non-living, was made up of these five basic elements. Therefore, matter is a composition of these five constituents.
Physical Nature of Matter:
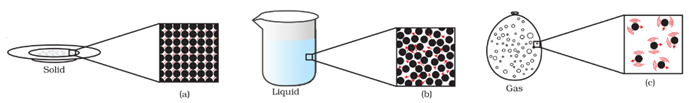
Matter is particulate in nature. This means that matter consists of particles.
See the microscopic image of a cube.
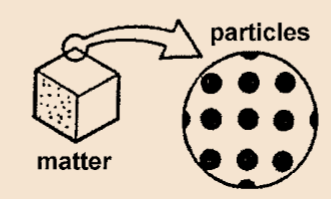
Example, If we put a drop of red color in water the color of the water turns red. This happens because the particles of red color mix with the particles of water.
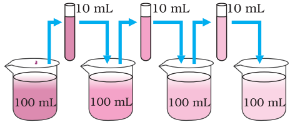
What is the size of these particles?
- The size of the particles of matter is very small.
- They are small beyond our imagination.
- They can be broken into further particles as well.
Example, On dilution of a colorful solution, as shown in the above figure, we can still see the color. This means there are millions of particles present in the color which just divide themselves on dilution.
Which of these is matter– happiness, air, sandwich, thoughts, juice, and eraser?
Air, sandwich, juice, and eraser as they have mass, they occupy space and can be broken into further particles.
[/responsivevoice]
[responsivevoice voice="UK English Female" rate="0.8" pitch="0.8" buttontext="Listen to this"]
Particles of matter have three characteristics:
- Particles of matter have spaces between them.
- Particles of matter are moving all the time.
- Particles of matter attract each other.
Particles of Matter have spaces between them :
Have you ever wondered what causes salt to get dissolved in water?
Salt gets dissolved in water because their particles have spaces between them. The particles of the salt get in between the spaces between the particles of water and a mixture is formed.
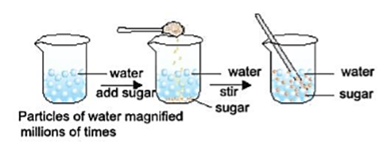
- We cannot see these particles through naked eyes.
Particles of Matter are continuously moving :
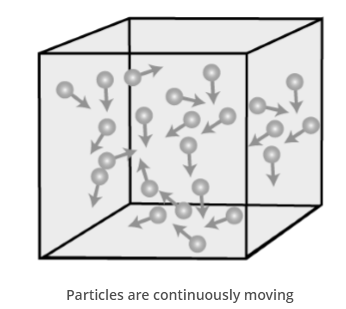
- Particles of matter are in motion all the time. Hence, they possess kinetic energy.
- Kinetic Energy– Energy due to motion
- The particles of a matter intermix on their own with other particles of a matter. Example, Salt in water, Various gases in the air, Ink in water.
- Diffusion– Diffusion is the process wherein the molecules merge as a result of their kinetic energy of random motion. It occurs in liquids and gases because their molecules move randomly. The molecules move from a region of higher concentration to a region of lower concentration, down the concentration gradient, until the concentration equalizes throughout the medium. Diffusion becomes faster on heating.
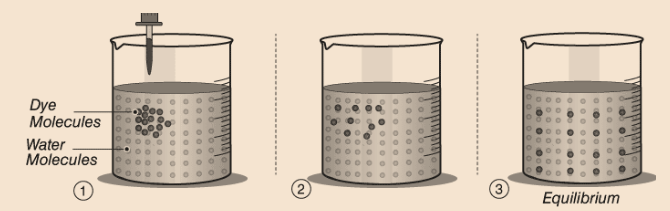
- The kinetic energy of particles also increases on heating.
Particles of Matter attract each other :
- The particles of matter are always held together because of a force of attraction between them.
- The amount of this force between the particles varies in different forms of matter, as shown in the figure below:
- Solids have the highest force of attraction. That is why we cannot move our hands through a solid object. The particles are so tightly bound.
- Similarly, particles of gases have the least force of attraction in them. We can move our hands easily in the air, can’t we? This is because the particles of air are loosely bound.
- We can arrange the force of attraction between different types of matter (solids, liquids, and gases) in increasing order as: Gas < Liquid < Solids
- We can also move our hands through water or liquid matter but not as freely as we can in the air. This means that they are also loosely bound to some extent. [/responsivevoice]
[responsivevoice voice="UK English Female" rate="0.8" pitch="0.8" buttontext="Listen to this"]
States of Matter :
Now we know that particles of matter have a force of attraction between them. Based on this criterion, we can say that matter is present in three different states: solid state, liquid state, and gaseous state.
The Solid State :
· Solids are the objects that have these three properties:
- They have a specific shape.
- They have distinct boundaries.
- They have a volume.
- There is less kinetic energy among the particles in solids. They are generally arranged in an order. Thus they possess a fixed shape. They cannot be compressed.
- The force of attraction is the maximum among the particles of solids. There is not much space between the particles. Therefore, they cannot be compressed.
Which of these are solids: Rubber band, Sponge, Salt?
All of them are solids. All of these follow the properties of solids. A rubber band and sponge change their shape only when we apply force on to them. It might appear to you as if salt is taking the shape of the container in which you put it but actually each of its grain has its own definite shape.
The Liquid State :
- Liquids have the following properties:
- Liquids have a fixed volume.
- Liquids do not have a fixed shape.
- The force of attraction in liquid particles is less than solids. Therefore, there is a space between the particles of liquids and they can flow easily. They cannot be compressed. That is why they are also called fluids.
- Particles of liquids arrange each other is not fixed. You might have seen that liquids take the shape of the container in which we put them. This is because the particles of liquids have a high kinetic energy, they always keep on moving.
Can other matter diffuse into liquids?
-
- Yes, other matter can diffuse into liquids whether it is solids, liquids, or gases. This is so because there is a space between the particles of liquid so particles of other matter can slip into those spaces.
- Diffusing solids into liquids: Mixing sugar in tea
- Diffusing liquids into liquids: Mixing ink in water
- Diffusing gases into liquids: The presence of oxygen and carbon dioxide in water
The Gaseous State :
- Gases have the following properties:
- They do not have a fixed volume.
- They do not have a fixed shape.
- The particles of gases have the least or almost no force of attraction between them. Therefore, the particles have a large number of spaces between them and they can freely move in any direction.
- Also, they can be easily compressed and put into a small container, unlike solids and liquids.
- Since there is a lot of space between the particles, different gases can diffuse into each other easily.
- The kinetic energy between the particles is the maximum in the case of gases. Therefore, the particles move around freely at high speed and there is no fixed shape of gases.
- Difference in the characteristic of states of matter
Solid Liquid Gas Definite shape Indefinite shape Indefinite shape Definite volume Definite volume Indefinite volume Maximum force of attraction between particles Less forces of attraction between particles compare to solid Negligible force of attraction between particles Cannot be compressed Cannot be compressed Can be compressed Kinetic energy of particles is minimum Kinetic energy of particles is more than solid Kinetic energy of particles is maximum Particles cannnot move rather they vibrate only at their fixed position Particles can slide over one another Particles can move freely Highest density Density is lower than solid Lowest denisty Cannot flow Flow Flow
Can Matter Change its State?
Water exists in three states:
-
- Ice – solid.
- Water – liquid.
- Water Vapor – Gas .
This is an indication that matter can change its states.
Effect of Change of Temperature :
What happens to matter when we heat it?
1. Solids:
-
-
- As we heat solids, the kinetic energy between the particles of solids increases which decreases the force of attraction between them.
- They start vibrating and changing their positions. Slowly, due to heat the particles become free and a solid converts into liquid.
- Melting Point– The temperature at which solid melts to become a liquid at atmospheric pressure. For Example, the melting point of ice is 273.16 Kelvin.
- Fusion– The process of melting of a solid into liquid is called Fusion.
-
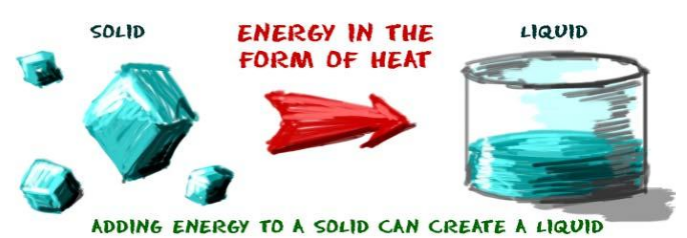
- In the melting process, once a solid reaches its melting point, its temperature does not increase further. So where does all the heat go? The heat present in the solid at time of melting is used by the particles to diminish the force of attraction between each other. The heat energy is therefore considered as hidden.
- Latent Heat– The heat energy which is used to break the force of attraction between the particles of matter is known as latent heat. Since the heat is hidden therefore it is called as Latent Heat.
- Latent Heat of Fusion– The amount of heat energy required to change 1 kg of a solid into liquid at atmospheric pressure at its melting point is known as the Latent Heat of Fusion.
- Atmospheric Pressure– Pressure exerted by the weight of the atmosphere.
2. Liquids:
- Just like in solids, the kinetic energy of particles of liquid increases, the force of attraction among them decreases and they start moving freely.
- As we keep on supplying the heat, a point comes when the particles overcome the forces of attraction completely.
- This is when a liquid starts changing into gas.

- Boiling Point- The temperature at which a liquid starts boiling at the atmospheric pressure is known as its Boiling Point. For Example, The boiling point of water is 373 Kelvin.
- Latent Heat of Vaporization – the amount of heat energy required to change 1 kg of a liquid into a gas at atmospheric pressure at its boiling point is known as Latent Heat of Vaporization.
What happens when we decrease the temperature?
1. Gases:
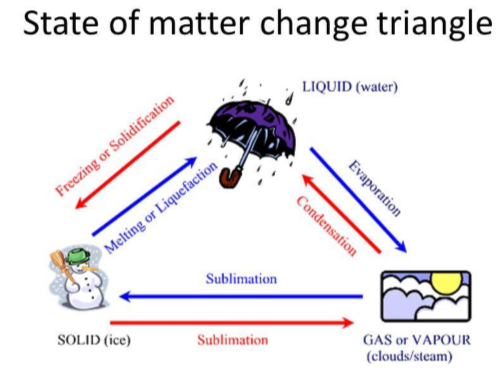
- The kinetic energy between the particles decreases and they turn into a liquid state.
- Condensation / Liquefaction– The process of converting a gas into a liquid by cooling down its temperature. For Example, The formation of clouds is due to condensation of water vapor from Earth.
2. Liquids:
- The kinetic energy between the particles decreases and they turn into a solid state. For Example, The formation of ice.
- Sublimation – change of state of a gas directly into solid and vice-versa is known as sublimation. Example, Camphor is a solid that directly evaporates into the air without changing to a liquid state.
Therefore, by increasing or decreasing the temperature we can change the states of matter into one another.
Effect of change of Pressure :
- By applying pressure, we can bring the particles of matter close to each other thereby, increasing the force of attraction among the particles.
- When we compress and decrease the temperature of a gas, the gas changes into a liquid.
- Dry Ice– Carbon dioxide in solid form is known as Dry Ice. It can directly turn into gas by decreasing the pressure to 1 atmosphere. [/responsivevoice]
[responsivevoice voice="UK English Female" rate="0.8" pitch="0.8" buttontext="Listen to this"]
Evaporation :
We already know that –
-
- Particles of matter are never at rest.
- Particles of matter possess different amounts of kinetic energy
- The particles of liquids have more kinetic energy. Therefore, they are able to overcome the forces of attraction and convert into vapor without any external forces.
- Evaporation– The phenomenon of change of a liquid into vapors at any given temperature below its boiling point is called Evaporation.
Evaporation is different than boiling, as shown in the figure below.
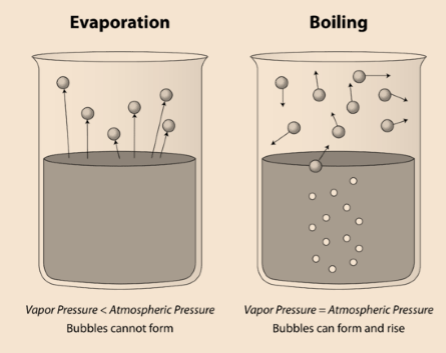
Factors Affecting Evaporation :
| Condition | Rate of Evaporation | Reason |
| Increase in Surface Area | Increases | Particles have more space and thus can evaporate easily |
| Increase in temperature | Increases | Kinetic energy among the particles increases |
| Increase in humidity | Decreases | Water content in air increases and so evaporation decreases |
| Increase in wind speed | Increases | Water vapours are blown away by winds allowing more evaporation |
How evaporation causes cooling?
The process of evaporation uses the energy of the liquid particles. Therefore, the particles absorb energy from the surroundings in order to compensate the energy that is being lost in the process of evaporation. This results in cooling of the surrounding area.

Example:
-
- Our palms feel cool when we put some acetone (nail paint remover) on it.
- People sprinkle water on their roofs or ground on sunny days to cool the area.
- We are able to sip hot tea faster in a saucer than in a cup.
Why people wear cotton clothes in summer?
We perspire more in summer. As the sweat evaporates it takes energy from our body surface and keeps our body cool. Cotton can absorb the sweat easily and exposes it to the atmosphere causing evaporation to take place easily. This, in turn, keeps us cool in summer days.
Why water droplets appear on the surroundings of a glass with ice-cold water?
There are water vapours present in the air. When they come in contact with the walls of the glass that has ice-cold water in it they condense. As a result, their state changes from gaseous state to liquid state thus forming tiny water droplets on the walls of the glass. [/responsivevoice]
[responsivevoice voice="UK English Female" rate="0.8" pitch="0.8" buttontext="Listen to this"]
The Five States of Matter
- By far we have discussed the three states of matter – Solid, Liquid, Gas.
- But, scientists have discovered that there are two more states of matter –
- Plasma
- Bose-Einstein Condensate
Plasma :
- It is a state of matter in which the particles are super excited and super energetic. They are in the form of ionized gases.
Example– Fluorescent tubes and neon light bulbs consist of plasma
- The neon bulbs contain neon gas and there is another gas such as helium in the fluorescent tube. As electricity is passed in the tube or the bulb, these gases get ionized and this creates the plasma inside them that glows.
In fact, the Sun and the stars glow because they plasma is present in them. Here are some examples of Plasma:
Bose-Einstein Condensate (BEC)
- It is the fifth state of matter discovered by Albert Einstein on the basis of the studies conducted by an Indian scientist Satyendra Nath Bose.
- BEC is formed by condensing gases of extremely low densities to much lower temperatures. [/responsivevoice]
[responsivevoice voice="UK English Female" rate="0.8" pitch="0.8" buttontext="Listen to this"]
Matter is made up of small particles.
• The matter around us exists in three states— solid, liquid and gas.
• The forces of attraction between the particles are maximum in solids, intermediate in liquids and minimum in gases.
• The spaces in between the constituent particles and kinetic energy of the particles are minimum in the case of solids, intermediate in liquids and maximum in gases.
• The arrangement of particles is most ordered in the case of solids, in the case of liquids layers of particles can slip and slide over each other while for gases, there is no order, particles just move about randomly.
• The states of matter are inter-convertible. The state of matter can be changed by changing temperature or pressure.
• Sublimation is the change of solid state directly to gaseous state without going through liquid state.
• Deposition is the change of gaseous state directly to solid state without going through liquid state.
• Boiling is a bulk phenomenon. Particles from the bulk (whole) of the liquid change into vapour state.
• Evaporation is a surface phenomenon. Particles from the surface gain enough energy to overcome the forces of attraction present in the liquid and change into the vapour state.
• The rate of evaporation depends upon the surface area exposed to the atmosphere, the temperature, the humidity and the wind speed.
• Evaporation causes cooling.
• Latent heat of vaporisation is the heat energy required to change 1 kg of a liquid to gas at atmospheric pressure at its boiling point.
• Latent heat of fusion is the amount of heat energy required to change 1 kg of solid into liquid at its melting point.
Important Measurement Units
| SI Unit of Mass | Kg (Kilogram) |
| SI unit of Volume | m3 (cubic meters) |
| Common unit of Volume | L (Liters) |
| SI unit of temperature | Kelvin 0O C = 273.16 K or 273 K (approximately) Kelvin = Celsius + 273 |
| Si unit of Pressure | Pa (Pascal) |
| For measuring pressure exerted by Gas | Atmosphere (atm) 1 atm = 1.01 X 105 Pa Normal Atmospheric Pressure = 1 atm (at sea level) |
[/responsivevoice]
NCERT-Class 9-Science-Chapter-1-Matter in our Surroundings -Exercise-Solution
Question 1:
Convert the following temperatures to Celsius scale.
(a) 300 K
(b) 573 K
Kelvin is an SI unit of temperature, where 0°C = 273.16 K (approximately 273 K) (a) 300 K = (300 − 273) °C = 27 °C (b) 573 K = (573 − 273) °C = 300 °C
Question 2:
Convert the following temperatures to Kelvin scale.
(a) 25°C
(b) 373°C
Kelvin is an SI unit of temperature, where 0°C = 273.16 K (approximately 273 K) (a) 25 °C = (25 + 273) K = 298 K (b) 373 °C = (373 + 273) K = 646 K
Question 3:
Give reason for the following observations.
(a) Naphthalene balls disappear with time without leaving any solid.
a) Some substances possess the property of sublimation like camphor and naphthalene balls. Such substances directly change from solid to gaseous state without changing into liquid like ice→ water→ water vapour does. Therefore, naphthalene balls disappear with time without leaving any solid.
(b) We can get the smell of perfume sitting several metres away.
b) Being a volatile substance (gets evaporated easily) perfumes change from liquid to gaseous state very fast. Those particles mix up with air particles and diffuse to reach our nostrils such that we get the smell of perfume sitting several metres away.
Question 4:
Arrange the following substances in increasing order of forces of attraction between particles − water, sugar, oxygen.
Sugar is a solid; the forces of attraction between the particles of sugar are strong. Water is a liquid; the forces of attraction here are weaker than sugar. Oxygen is a gas; the forces of attraction are the weakest in gases. Thus, the increasing order of forces of attraction between the particles of water, sugar and oxygen is Oxygen < water< sugar
Question 5:
What is the physical state of water at
(a) 25°C
(b) 0°C
(c) 100°C
At 25 C water is liquid, at 0 C water is solid(ice), at 100 C water exists as both liquid and gas.
Question 6:
Give two reasons to justify−
(a) water at room temperature is a liquid.
(a) At room temperature (25 °C), water is a liquid because it has the following characteristic of liquid: (i) At room temperature, water has no shape but has a fixed volume that is, it occupies the shape of the container in which it is kept. (ii) At room temperature, water flows.
(b) an iron almirah is a solid at room temperature.
(b) An iron almirah is a solid at room temperature (25 °C) because: (i) it has a definite shape and volume like a solid at room temperature. (ii) it is rigid as solid at room temperature.
Question 7:
Why is ice at 273 K more effective in cooling than water at the same temperature?
Ice at 273 K has less energy than water (although both are at the same temperature). Water possesses the additional latent heat of fusion. Hence, at 273 K, ice is more effective in cooling than water.
Question 8:
What produces more severe burns, boiling water or steam?
Steam at 373K has more heat energy equal to the latent heat of vaporisation than boiling water at 373K,therefore, steam produces more severe burns than boiling water.
Question 9:
Name A, B, C, D, E and F in the following diagram showing change in its state.
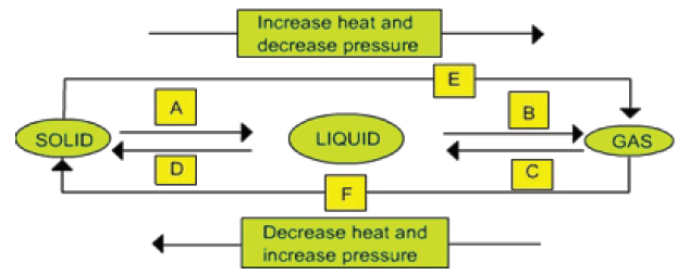
A – Fusion (Heating – Melting) B – Vaporisation C – Cooling – Condensation (Liquefaction) D – Cooling – Freezing (Solidification) E – Sublimation F – Sublimation
Question 1.1:
Which of the following are matter?
Chair, air, love, smell, hate, almonds, thought, cold, cold drink, smell of perfume.
Anything that occupies space and has mass is called matter. Matter can exist in three physical states—solid, liquid, and gaseous. Chair and almond are forms of matter in the solid state. Cold drink is a liquid state of matter. Air and smell of perfume are gaseous states of matter. Note: The sense of smell is not matter. However, the smell or odour of a substance is classified as matter. The smell of any substance (say, perfume) is the gaseous form of that substance which our olfactory system can detect (even at very low concentrations). Hence, smell of perfume is matter.
Question 1.2:
The smell of hot sizzling food reaches you several metres away, but to get the smell from cold food you have to go close.
Since hot sizzling food has temperature higher than cold food and at higher temperature diffusion rate (movement) of particles is very fast, due to this the smell of hot sizzling food reaches us from several metres away.
Question 1.3:
A diver is able to cut through water in a swimming pool. Which property of matter does this observation show?
The ability of a diver to cut through water in a swimming pool shows that matter is made up of particles.
Question 1.4:
What are the characteristics of particles of matter?
The characteristics of particles of matter are as follows: i) Particles of matter have spaces between them. ii) Particles of matter are in continuous motion. iii) Particles of matter have an attractive force between them to keep them together.
Question 2.1:
The mass per unit volume of a substance is called density (density = mass/volume).
Arrange the following in order of increasing density − air, exhaust from chimney, honey, water, chalk, cotton, and iron.
The given substances in the increasing order of their densities can be represented as: Air < Exhaust from chimney < Cotton < Water < Honey < Chalk < Iron
Question 2.2:
(a) Tabulate the differences in the characteristics of states of matter.
Solid
Liquid
Gas
Definite shape
Indefinite shape
Indefinite shape
Definite volume
Definite volume
Indefinite volume
Maximum force of attraction between particles
Less forces of attraction between particles compare to solid
Negligible force of attraction between particles
Cannot be compressed
Cannot be compressed
Can be compressed
Kinetic energy of particles is minimum
Kinetic energy of particles is more than solid
Kinetic energy of particles is maximum
Particles cannnot move rather they vibrate only at their fixed position
Particles can slide over one another
Particles can move freely
Highest density
Density is lower than solid
Lowest denisty
Cannot flow
Flow
Flow
(b) Comment upon the following: rigidity, compressibility, fluidity, filling a gas container, shape, kinetic energy, and density.
(b) Rigidity→ It is the property of matter to maintain its shape even if external forces work and the solids show this property. Compressibility → It is the property of matter to allow decrease in volume under high pressure and the gases show this property. Fluidity → It is the property of a substance to easily flow and allow change in its shape under external forces and this property is exhibited by both liquids and gases. Filling a gas container → Gases can be compressed easily hence they can be filled within a vessel at high pressure. This property of gases allows their convenient filling into a small container or cylinder and that also in a large volume. It also allows their easy transport from one place to the other e.g. CNG. Shape→ According to the type of matter shape differs depending upon location of particles like solids have definite shape while liquids acquire the shape of their container and gases as such don’t have any shape. Kinetic energy → It is the kind of energy present in an object when it is under motion as the particles of that object/matter are continuously moving therefore matter has kinetic energy. However greater is the movement more will be the kinetic energy and vice-a-versa i.e. solid < liquid < gas. Density → Mass per unit volume of a substance/matter is known as its density i.e. density = mass/volume
Question 2.3:
Give reasons:
(a) A gas fills completely the vessel in which it is kept.
(b) A gas exerts pressure on the walls of the container.
(c) A wooden table should be called a solid.
(d) We can easily move our hand in air, but to do the same through a solid block of wood, we need a karate expert.
(a) There is little attraction between particles of gas. Thus, gas particles move freely in all directions. Therefore, gas completely fills the vessel in which it is kept. (b) The gas particles are in random motion due to weak intermolecular force of attraction. These gaseous molecules continuously collide among themselves and they hit the walls of the container with a greater force. Therefore, gas exerts pressure on the walls of the container. (c) A wooden table has a definite shape and volume. It is very rigid and cannot be compressed i.e., it has the characteristics of a solid. Hence, a wooden table should be called a solid. (d) Particles of air have large spaces between them. On the other hand, wood has little space between its particles. Also, it is rigid. For this reason, we can easily move our hands in air, but to do the same through a solid block of wood, we need a karate expert.
Question 2.4:
Liquids generally have lower density as compared to solids. But you must have observed that ice floats on water. Find out why.
When water freezes to form ice,some empty spaces are created. As a result,volume increases for the same mass of water. In other words, mass per unit volume or density of ice is lower than that of water and hence ice floats over water.
Question 3.1:
Convert the following temperature to Celsius scale:
(a) 300 K
(b) 573 K
(a) 300 K = (300 − 273)°C = 27°C (b)573 K = (573 − 273)°C = 300°C
Question 3.2:
What is the physical state of water at:
(a) 250°C
(b) 100°C
(a) 100 C is the boiling point of water. Therefore, at 250 C i.e. at a temperature higher than its boiling point is gaseous. (b) At 100 C, the boiling point of water, water exists both as a liquid as well as a gas.
Question 3.3:
For any substance, why does the temperature remain constant during the change of state?
During a change of state, the temperature remains constant. This is because all the heat supplied to increase the temperature is utilised in changing the state by overcoming the forces of attraction between the particles. Therefore, this heat does not contribute in increasing the temperature of the substance.
Question 3.4:
Suggest a method to liquefy atmospheric gases.
By applying pressure and reducing the temperature, atmospheric gases can be liquefied.
Question 4.1:
Why does a desert cooler cool better on a hot dry day?
When a liquid evaporates, the particles of the liquid absorb energy from the surroundings to compensate the loss of energy during evaporation. This makes the surroundings cool. In a desert cooler, the water inside it is made to evaporate. This leads to absorption of energy from the surroundings, thereby cooling the surroundings. Again, we know that evaporation depends on the amount of water vapour present in air (humidity). If the amount of water vapour present in air is less, then evaporation is more. On a hot dry day, the amount of water vapour present in air is less. Thus, water present inside the desert cooler evaporates more, thereby cooling the surroundings more. That is why a desert cooler cools better on a hot dry day.
Question 4.2:
How does water kept in an earthen pot (matka) become cool during summers?
There are some pores in an earthen pot through which the liquid inside the pot evaporates. This evaporation makes the water inside the pot cool. In this way, water kept in an earthen pot becomes cool during summers.
Question 4.3:
Why does our palm feel cold when we put some acetone or petrol or perfume on it?
Organic compounds are covalently bonded and are volatile in nature. When we put some acetone or petrol or perfume on our palm, it evaporates. During evaporation, particles of the liquid absorb energy from the surrounding or the surface of the palm to compensate for the loss of energy, making the surroundings cool. Hence, our palm feels cold when we put some acetone or petrol or perfume on it.
Question 4.4:
Why are we able to sip hot tea or milk faster from a saucer than a cup?
A liquid has a larger surface area in a saucer than in a cup. Thus, it evaporates faster and cools faster in a saucer than in a cup. For this reason, we are able to sip hot tea or milk faster from a saucer than a cup.
Question 4.5:
What type of clothes should we wear in summers?
We should wear cotton clothes in summers. During summers, we sweat more. On the other hand, cotton is a good absorber of water. Thus, it absorbs sweat from our body and exposes the liquid to the atmosphere, making evaporation faster. During this evaporation, particles on the surface of the liquid gain energy from our body surface, making the body cool.
NCERT-Class 9-Science-Chapter-1-Matter in our Surroundings-Videos
Click on link to open video
1- [video_lightbox_youtube video_id="uUtmLNouDOg&rel=0" width="640" height="520" start="10" anchor="Full Chapter"]