Metallurgy
Class 10-Science & Technology Part-1-Chapter-8- Maharashtra Board
Notes
|
Topics to be learn :
|
Physical properties of metals and nonmetals :
Properties of metals:
(i) Solid state: Under ordinary conditions, metals are generally solids.
- (Exception : The metals namely, mercury and gallium exist in liquid state at room temperature.)
(ii) Typical lustre : Metals usually have a high lustre called as metallic lustre. They can be polished to give a highly reflective surface.
(iii) Malleability and ductility : Metals are ductile and malleable.
- The property due to which a substance can be drawn into a thin wire without cracking or breaking is called ductility. Metals are ductile. Thus, a metal can be drawn into a wire.
- The property due to which a substance can be hammered or rolled into a thin sheet without cracking is called malleability. Metals are malleable. Thus, a metal can be hammered to form a thin sheet.
(iv) Hardness : Metals are usually hard, but not brittle.
- (Exception : Lithium, sodium and potassium)
(v) Good conductors of heat and electricity : Metals are good conductors of heat and electricity.
- The electrons in the outermost shells of atoms of a metal are free to move throughout the metal. When a metal is heated, these electrons start moving with higher velocity and conduct heat. Hence, metals are good conductors of heat.
- The electrons in the outermost shells of atoms of a metal are free to move throughout the metal. When a potential difference is applied between the ends of a metal wire, the net movement of the electrons in a particular direction, from a point at lower potential to a point at higher potential, constitutes an electric current. Hence, metals are good conductors of electricity.
(vi) High melting and boiling points : (Exception : the melting and boiling points of the metals sodium, potassium, mercury and galium are very low.)
(vii) Sonorous and produce sound on striking a hard surface.
Properties of nonmetals :
(i) Gaseous or solid state : (Exception : nonmetal bromine which exists in liquid state.)
(ii) Lack of any typical lustre : (Exception : Iodine and Diamond)
(iii) Brittleness in the solid state : (Exception : Diamond is the hardest natural substance)
(iv) Bad conductors of heat and electricity : (Exception : Graphite) (Diamond is good conductor of heat)
(v) Low melting and boiling points :
Chemical properties of metals :
Reactions of Metals :
(i) Reaction of metals with oxygen : Metals combine with oxygen on heating in air and metal oxides are formed.
- Sodium and potassium are highly reactive metals. Sodium metal combines with oxygen in the air at room temperature to form sodium oxide.
4Na(s) + O2 (g) → 2Na2O(s)
- Sodium readily catches fire on keeping exposed to air producing a lot of heat. Therefore, to prevent accident in the laboratory or elsewhere it is kept in Kerosene.
- Sodium oxide reacts with water to form sodium hydroxide (alkali)
Na2O (s) + H2O (l) → 2NaOH (aq)
- Magnesium ribbon burns in air form magnesium oxide.
2Mg(s) + O2 (g) → 2MgO(s)
- Magnesium oxide reacts with water to form magnesium hydroxide.
MgO + H2O → Mg(OH)2
(ii) Reaction of metals with water :
- Sodium and potassium react vigorously with water to evolve hydrogen.
2Na (s) + 2H2O (l) → 2NaOH (aq) + H2(g) + heat
2K(s) + 2H2O (l) → 2KOH (aq) + H2(g) + heat
- Calcium reacts with water less vigorously to evolve hydrogen.
2Ca(s) + 2H2O (l) → 2Ca(OH)2(aq) + H2(g)
- Magnesium reacts with hot water to evolve hydrogen.
Mg(s) + 2H2O(hot) → Mg(OH)2(aq) + H2(g)
- Aluminium, iron and zinc do not react with cold or hot water but they react with steam to evolve their oxides and hydrogen.
2Al(s) + 3H2O(g) → Al2O3 (s) + 3H2(g)
3Fe(s) + 4H2O(g) → Fe3O4(s) + 4H2(g)
Zn(s) + H2O(g) → ZnO(s) + H2(g)
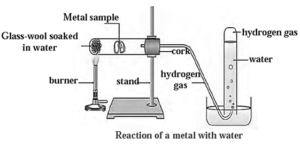
(iii) Reaction of metals with acids: Metals react with dilute hydrochloric acid or dilute sulphuric acid to form metal chloride or metal sulphate and hydrogen gas. The rate of evolution of H2 is maximum in case of magnesium. The reactivity decreases in the order Mg > Al > Zn > Fe.
Mg(s) + 2HCl (aq) → MgCl2(aq) + H2(g)
2Al (s) + 6HCl (aq) → 2AlCl3(aq) +3H2(g)
Fe(s) + 2HCl (aq) → FeCl2(aq) + H2(g)
Zn (s) + HCl (aq) → ZnCl2(aq) + H2(g)
Fe(s) + H2SO4(aq) → FeSO4(aq) + H2(g)
Zn(s) + H2SO4(aq) → ZNSO4(aq) + H2(g)
Mg(s) + H2SO4(aq) → MgSO4(aq) + H2(g)
Zn(s) + H2SO4(aq) → ZNSO4(aq) + H2(g)
(iv) Reaction of metals with nitric acid : Metals react with nitric acid to form nitrate salts. Depending on the concentration of nitric acid, various oxides of nitrogen (N2O NO, NO2) are formed.
Aqua Regia : Aqua Regia is freshly prepared by mixing concentrated hydrochloric acid and concentrated nitric acid in the ratio 3:1. Aqua Regia is a highly corrosive and fuming liquid. It is one of the few reagents which can dissolve the noble metals like gold and platinum.
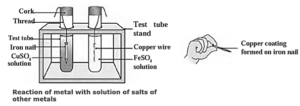
(v) Reactions of metals with salts of other metals : The reactivity of all metals is not the same. All metals do not react with oxygen, water and acids. As a result, the relative reactivity of metals cannot be determined using these reagents. If a metal A displaces another metal B from the solution of its salt, it means that the metal A is more reactive than the metal B.
- Metal A + Salt solution of metal B → Salt solution of metal A + Metal B.
Reactivity series of Metals :
Reactivity series of Metals:
The arrangement of metals in the increasing or decreasing order of reactivity is called the reactivity series of metals.
Metals are divided into the following groups according to their reactivity.
- Highly reactive metal.
- Moderately reactive metals.
- Less reactive metals.
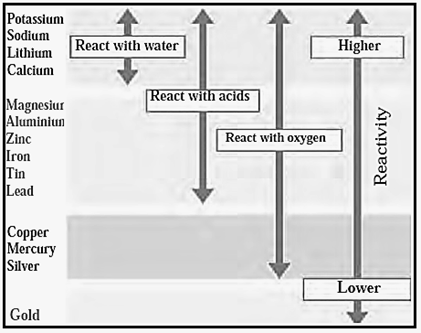
(a) Highly reactive metal :
- The metals which are placed at the top of reactivity series are very reactive.
- They are never found in nature as free elements. g., sodium, potassium, calcium and aluminium.
- These metals are obtained by electrolytic reduction.
(b) Moderately reactive metals :
- The metals in the middle of reactivity series such as iron, zinc, lead, copper are moderately reactive.
- These elements are present as sulphides or carbonates in nature.
- Generally metals are obtained from their oxide as compared to their sulphides and carbonates.
(c) Less reactive metals :
- The metals which are placed at the bottom of the reactivity series are least reactive.
- They occur in free state, e.g. gold, silver and copper.
- Copper and silver are also found in the combined state as sulphide and oxide ores.
- These metals are obtained from their ores by just heating the ores in air.
(vi) Reaction of metals with nonmetals : The ionic compound is formed when metal reacts with nonmetal. Ionic compound is formed as sodium loses one electron while chlorine accepts one electron.
2Na + Cl2 → 2NaCl (sodium chloride)
(Metal) (Nonmetal) (Ionic compound)
Chemical properties of nonmetals :
(i) Reaction of nonmetals with oxygen : Non-metals combine with oxygen to form acidic oxides. In some cases, neutral oxides are formed.
C + O2 CO2 (Acidic)
2C + O2 2CO(Neutral)
S + O2 SO2 (Acidic)
(ii) Reaction of nonmetals with water : Non-metals do not react with water, (Exception—Halogen). Chlorine dissolves in water giving the following reaction.
Cl2(g) + H2O(l) → HOCl(aq) + HCl(aq)
(iii) Reaction of dilute acid with nonmetals : Nonmetals do not react with dilute acids, (Exception—Halogen). Chlorine reacts with dilute hydrobromic acid to form bromine and HCl.
Cl2(g) + 2HBr(aq) → 2HCl(aq) + Br2(aq)
(iv) Reaction of nonmetals with hydrogen : Nonmetals react with hydrogen under certain condition (such as proper temperature, pressure, use of catalyst, etc.)
S + H2 → H2S
N2 + 3H2 → 2NH3
Ionic compounds :
Ionic compounds : The compounds formed from two units, ie. cation and anion are called ionic compounds.
- An electrostatic force of attraction between oppositely charged ions (cations and anions) is called an ionic bond.
General properties of ionic compounds :
- Ionic compounds are crystalline solids have a definite shape and hard due to strong electrostatic force of attraction between oppositely charged ions.
- They are generally brittle. When pressure is applied they break into pieces.
- They have high melting and boiling points.
- They are soluble in water and insoluble in solvents such as Kerosene and petrol.
- They conduct electricity in the molten state and also in an aqueous solution.
Metallurgy :
The process of extraction of metal in pure form from the ores. The metals are further purified by different methods of purification. All this process is called metallurgy.
Minerals and ores :
Minerals : The compounds of metals that occur in nature along with the impurities are called minerals.
Examples:
- Roks : are composed of mixtures of minerals Talc and granite are minerals.
- Gangue : Ores contain metal compounds with some of the impurities like soil, sand, rocky materials etc. These impurities are called gangue.,
Ores: The minerals from which metals are extracted profitably and conveniently are called ores.
Examples: Bauxite (AL2O3, FEO). Cinnabar (HgS).
Basic principles of metallurgy : Pure metal is obtained from the ore by the following stages.
Stage 1- Concentration of ores: The process of separating gangue from the ores is called concentration of ores.
Methods of concentration of ores:
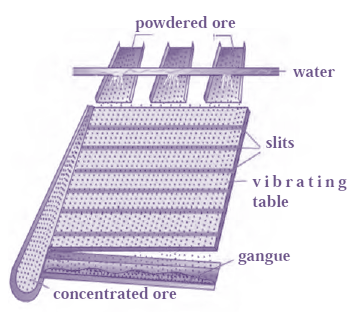
(a) Separation based on gravitation : The gravitational method is used to separate the heavy particles of ores from the light particles of gangue. The processes to do this separation are as follows.
(i) Wilfley table method :
(i) Wilfley table method (Separation based on gravitation) :
- The Wilfley table is made by fixing narrow and thin wooden wedges / blocks on inclined surface with low slope.
- The table is kept continuously vibrating.
- Lumps of the ore is made powdered ore by using ball mill.
- This powdered ore is poured on the table and a stream of water is simultaneously released from the upper side.
- This result in the lighter gangue particles getting carried away along with the flowing water,
- While The heavier particles, which have a higher proportion of minerals, are blocked by the wooden wedges and collected through slits.
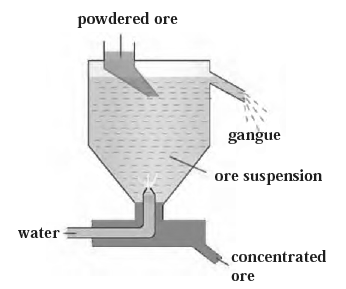
(i) Hydraulic separation method :
(ii) Hydraulic separation method : The hydraulic separation method is based on the working of a mill.
- The hydraulic separation method is a method used to separate finely ground ore from the rest of the ore.
- It involves adding finely ground ore to a tapering vessel, which opens in a tank-like container with an outlet for water and a water inlet.
- A fast stream of water is released in the tank from the lower side.
- The lighter gangue particles flow out along with the water stream from the outlet on the upper side of the tank and are collected separately.
- Simultaneously the heavy particles of the ore are collected at the bottom from the lower side of the tank.
- This method is based on the law of gravitation, Where in particles of the same size are separated by their weight with the help of water.
(b) Magnetic separation method:
Electro-magnetic machine is used in this method. The main parts of this machine are two types of iron rollers and the conveyor belt continuously moving around them.
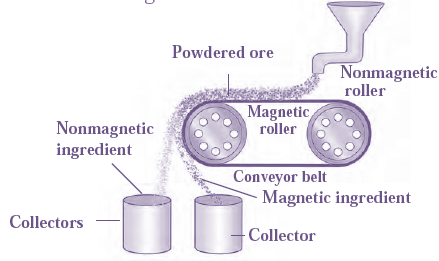
Magnetic Separation Method Overview :
- One roller is nonmagnetic, the other is electromagnetic.
- The conveyor belt is made of nonmagnetic leather or brass.
- Powdered ore is poured at the nonmagnetic roller end of the conveyor belt.
- Two collector vessels are placed below the magnetic roller.
- Nonmagnetic ore particles are carried along the belt and fall in the collector vessel.
- Magnetic ore particles stick to the magnetic roller and fall in the collector vessel near the belt.
In this way, the magnetic and nonmagnetic particles in the ore are separated because of their magnetic nature.
Example : Cassiterite is a tin ore. It contains mainly the nonmagnetic ingredient stannic oxide (SnO2) and the magnetic ingredient ferrous tungstate (FeWO4). These are separated by the electromagnetic method.
(c) Froth floatation method: The froth floatation method is a process that involves combining finely ground mineral with vegetable oil, such as pine oil or eucalyptus oil, to create a froth. The hydrophobic properties of the particles cause the metal sulphides particles to wet primarily with oil, while the hydrophilic properties cause the gangue particles to wet with water.
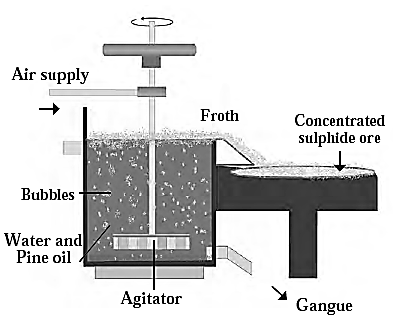
Froth Flotation Process :
- Pressurised air is blown through mixture.
- An agitator rotates around the tank's axis.
- Bubbles form due to blown air.
- Foam from oil, water, and air bubbles forms.
- Foam rises to water surface and floats.
- Sulphide minerals float with foam, wet by oil and can be removed.
- Gangue particles settle at the bottom.
- Used for concentration of zinc blend (ZnS) and copper pyrite (CuFeS2).
(d) Leaching :
- Leaching is the initial step in extracting metals like aluminium, gold, and silver from ores.
- The ore is soaked in a specific solution for a long time, causing it to dissolve.
- The gangue doesn't react, allowing easy separation.
- For example, bauxite concentration is achieved by soaking it in aqueous NaOH or Na2CO3, causing it to dissolve alumina.
Stage 2-Extraction :
(a) Extraction of reactive metals :
The extraction of highly reactive metals has to be done by electrolytic reduction.
- Reactive Metal Extraction Overview
Highly reactive metals like potassium, sodium, and aluminium are extracted. - They form cations by losing electrons in their outermost shell.
- Reactive metals react vigorously with dilute acids to give hydrogen gas.
- Highly reactive metals burn by reacting with oxygen from air.
- For example, the metals sodium, calcium and magnesium are obtained by electrolysis of their molten chloride salts.
- Metal is deposited on the cathode, while chlorine gas is liberated at the anode.
The electrode reactions during the electrolysis of molten sodium chloride to get metallic sodium are as shown below.
Cathode reaction Na+ + e- → Na (Reduction)
Anode reaction 2Cl— → Cl2 + 2e- (Oxidation)
Extraction of Aluminium :
Extraction of Aluminium :
Atomic number : 13, Electronic configuration : 2,8,3, Valency : 3.
Aluminium is extracted from its main ore bauxite (Al2O3.nH2O).
Extraction of aluminium involves the following steps :
- Concentration of bauxite ore (Bayer’s process)
- Electrolytic reduction of pure alumina
(i) Concentration of bauxite ore :
Bauxite Ore Concentration Process :
- Powdered bauxite heated with NaOH at 140°C to 150°C for 2-8 hours.
- Al2O3 forms sodium aluminate (NaAlO2), water-soluble.
- Iron oxide impurities separate by filtration.
- Silica reacts with sodium hydroxide to form sodium silicate.
- NaAlO2 hydrolyzed to form insoluble Al(OH)3.
- Aluminium hydroxide precipitate is then filtered, dried and calcinated by heating at 1000°C to obtain pure alumina.
Al2O3 + 2NaOH → 2NaAlO2 + H2O
NaAlO2 + 2H2O → NaOH + Al(OH)3
2 Al(OH)3 Al2O3 + 3 H2O
(ii)Electrolytic reduction of alumina:
- In this method electrolysis of molten mixture of alumina (melting point > 2000 0C) is done in a steel tank.
- The tank has a graphite lining on the inner side. This lining does the work of a cathode.
- A set of graphite rods dipped in the molten electrolyte works as anode.
- Cryolite (Na3AlF6) and fluorspar (CaF2) are added in the mixture to lower its melting point up to 1000 0
- Aluminium is deposited on the cathode on passing electric current.
- Heavier molten aluminium is collected at the bottom of the tank and periodically removing it.
- Oxygen gas is liberated at the anode.
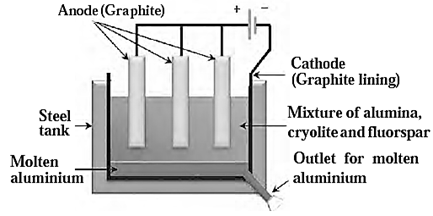
Reactions :
Al2O3 → 2Al3+ + 3O2-
(fused)
At the anode : 2O2 → O2 + 4e-
At the cathode: Al3+ + 3e- → Al
(b) Extraction of moderately reactive metals :
- In metals, in the middles of the reactivity series such as iron, zinc, lead, copper are moderately reactive.
- These metals occur in the form of their sulphide salts or carbonate salts.
- The sulphides ores are strongly heated in air to convert them into oxides. This process is called roasting.
- Carbonate ores are strongly heated in a limited supply of air to convert them into oxides. This process is called
The following reaction occur during roasting and cacination of zinc ore.
Roasting :2ZnS+3O2 → 2ZnO +2SO2
Calcination : ZnCO3 → ZnO+CO2
The zinc oxide is reduced to zinc by using suitable reductant such as carbon.
ZnO +C → Zn + CO
(c) Extraction of less reactive metals :
- The less reactive metals are at the bottom of reactivity series of metal.
- These metals are found in free state in nature. For example, gold, silver, platinum.
- The reserves of copper in free state are very few. Presently copper is found mainly in the form of Cu2
Copper is obtained from Cu2S ore just by heating in air.
2Cu2S +3O2 → 2Cu2O + 2SO2
2Cu2O + Cu2S → 6Cu + SO2
Stage 3- Refining of metals : Metals Obtained by various reduction processes contain impurities, Electrolysis method is used to remove impurities to obtain pure metals.
Corrosion of metals :
Corrosion : Corrosion is a natural process that occurs when a metal reacts with its environment and gradually deteriorates
- It is an oxidation process that occurs in the presence of oxygen.
- Metals higher in the reactivity series (like iron and zinc) corrode more easily, while those lower in the series (like gold, platinum, and palladium) are less prone to corrosion.
Examples :
- Iron reacts with moist air and a deposit of reddish substance (Fe2O3.H2O) is formed on it. This substance is called rust.
- Carbon dioxide in moist air reacts with the surface of copper vessel. Copper loses its luster due to formation of greenish layer of copper carbonate (CuCO3) on its surface. This is called patination of copper.
- On exposure to air, silver articles turn blackish after some time. This is because
- of the layer of silver sulphide (Ag2S) formed by the reaction of silver with hydrogen sulphide in air.
- By oxidation of aluminium, a thin layer of aluminium oxide forms on it.
Prevention of corrosion :
- Metals are protected from corrosion through various methods, with a particular focus on preventing iron rust.
- To reduce the rate of rusting, metals should be isolated from direct air contact.
- Prevention methods include fixing a substance on the metal surface to prevent contact with moisture and oxygen in the air, and applying a layer of paint, oil, grease, or varnish on their surface. This method, for instance, can prevent iron corrosion.
- Overall, preventing corrosion is crucial for maintaining the longevity and quality of metals.
Corrosion can be prevented by putting a layer of noncorrodible metal on a corrodible metal. This can be done in many ways.
Corrosion prevention methods :
(i) Galvanizing.
- The process of coating a thin layer of zinc on iron or steel is called galvanization.
- In galvanization and iron object is dipped into molten zinc.
- A thin layer of zinc is formed all over the from object.
- Examples : Shiny iron nails, pin, iron pipes,
(ii) Tinning :
- The process of coating a thin layer of tin (molten tin) on copper or brass is called tinning.
- Cooking vessels made of copper and brass get a greenish coating due to corrosion, the greenish substance is copper carbonate and it is poisonous. Therefore, these vessels are coated with tin to prevent corrosion (Kalhaee).
(iii) Anodization:
- The anodising technique is an application of electrolysis,
- In this method metals Hike copper and aluminium are coated with a thin and strong layer of their oxides by means of electrolysis.
- This thin film protects the metals from corrosion.
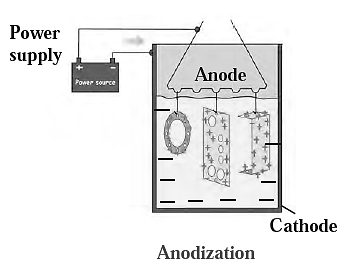
Example : Anodizing aluminium creates a thin layer of aluminium oxide, preventing further oxidation by obstructing oxygen and water contact. This protection can be enhanced by increasing the thickness of the oxide layer.
Uses : It is used for manufacturing kitchen articles such as pressure cooker and anodized pan.
(iii) Electroplating :
The electrolytic process of depositing less reactive metal, generally a superior metal like silver, chromium or nickel, over another metal is called electroplating.
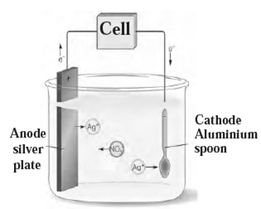
Examples: Silver plated spoon, gold plated jewellery.
(iv) Alloying :
- A homogenous mixture of two or more metals or a metal and a nonmetal in a definite proportion is called an alloy.
- The physical properties of an alloy are different from those of its constituents. Alloys are corrosion resistant.
- Examples : Brass is made from copper and Zinc. Copper and tin are used to make an alloy called bronze. Stainless steel is made from iron, carbon and chromium.
Know This :
- When one of the metals in an alloy is mercury the alloy is called amalgam. For example, sodium amalgam, zinc amalgam, etc. Silver amalgam was earlier used by dentists. Gold amalgam is used for extraction of gold.
- When a metal loses electrons the process is called an oxidation while when a nonmetal gains electrons, it is called a reduction.
Na → Na++e- (oxidation)
Cl+e-→ Cl- (reduction)
Various alloys used in daily life :
Various alloys used in daily life :
| Alloys | Uses |
| Steel: | Construction: Used in buildings, bridges, and skyscrapers due to its strength and durability.
Automobiles: Car frames, engine components, and wheels. Utensils: Stainless steel kitchenware. Tools: Wrenches, hammers, and saws. |
| Brass | Plumbing: Faucets, valves, and pipes.
Musical Instruments: Trumpets, trombones, and saxophones. Decorative Hardware: Door handles and ornaments. |
| Bronze: | Statues and Medals: Bronze is commonly used for artistic sculptures.
Bearings and Bushings: Due to its low friction properties. Historical Artifacts: Ancient coins and tools. |
| Aluminum Alloys: | Aircraft Construction: Lightweight and strong.
Bicycles and Sports Equipment: Frames and components. Packaging: Aluminum foil and cans. |
| Titanium Alloys | Aerospace: Aircraft parts and spacecraft components.
Medical Implants: Biocompatible and corrosion-resistant. Sports Equipment: Golf clubs and bicycle frames. |
| Gold Alloys: | Jewelry: 22-karat gold for durability.
Electronics: Gold-plated connectors for conductivity. |
| Nichrome: | Heating Elements: Used in toasters, hair dryers, and electric stoves.
Resistance Wires: High electrical resistance. |
Useful links :
| Main Page : - Maharashtra Board Class 10 Science & Technology Part-1,Part-2 - All chapters notes, solutions, videos, test, pdf.
Books : MSBSHSE -Class 10th Science Text Books – Chapter wise PDF for download Previous Chapter : Chapter 7- Lenses - Online Notes Next Chapter : Chapter 9 -Carbon Compounds - Online Notes |
