Metallurgy
Class 10-Science & Technology Part-1-Chapter-8- Maharashtra Board syllabus
Solution
Question 1:
Write names.
i. Alloy of sodium with mercury.
Sodium amalgam, commonly denoted as Na(Hg), it is an alloy of mercury and sodium.
ii. Molecular formula of the common ore of aluminium.
Al2O3.nH2O.
iii. The oxide that forms salt and water by reacting with both acid and base.
Aluminium Oxide ( Al2O3 ) can react both an acid as well as a base to produce salt and water.
iv. The device used for grinding an ore.
The device used for grinding an ore is grinding mill.
v. The nonmetal having electrical conductivity.
Graphite is a good conductor of electricity.
vi. The reagent that dissolves noble metals.
Aqua regia is 1:3 mixture of concentrated nitric and hydrochloric acids. It dissolves noble metals such as gold, and platinum.
Question 2:
Make pairs of substances and their properties
|
Substance |
Property |
| a. Potassium bromide | 1. Combustible |
| b. Gold | 2. Soluble in water |
| c. Sulphur | 3. No chemical reaction |
| d. Neon | 4. High ductility. |
Substance Property
a. Potassium bromide
2. Soluble in water
b. Gold
4. High ductility
c. Sulphur
1. Combustible
d. Neon
3. No chemical reaction
Question 3:
Identify the pairs of metals and their ores from the following.
|
Group A |
Group B |
| a. Bauxite | i. Mercury |
| b. Cassiterite | ii. Aluminium |
| c. Cinnabar | iii. Tin |
Group A Group B
a. Bauxite
i. Aluminium
b. Cassiterite
ii. Tin
c. Cinnabar
iii. Mercury
Question 4:
Explain the terms.
1. Metallurgy
Metallurgy: The process used for extraction of metals in their pure form from its ores and then metals are further purified by various methods of purification. All the process is called metallurgy.
The major steps involved for the extraction of a metal from its ore are:
(i) Concentration of ores (or enrichment of ore)
(ii) Conversion of concentrated ore into metal
(iii) Refining (purification) of impure metal
2. Ores
Ores: Those minerals from which a metals can be extracted conveniently and profitably. Example : Bauxite (Al2O3.H2O.) Cinnabar (HgS)
3. Minerals.
Minerals: The Naturally occurring compounds of metals along with other impurities are known as minerals. Example :Rocks are composed of mixtures of minerals. Talc and granite are minerals.
d. Gangue
Gangue is the unwanted impurities like rock material, dust, soil, sand, earthy particles, limestone, mica etc. present in an ore.
Question 5:
Write scientific reasons.
a. Lemon or tamarind is used for cleaning copper vessels turned greenish.
b. Generally the ionic compounds have high melting points.
In an ionic compound there is strong electrostatic force of attraction between opposite charged ions. To overcome these forces a considerable amount of energy is needed. Therefore, ionic compounds have high melting points.
c. Sodium is always kept in kerosene.
Sodium is a very reactive metal. It vigorously reacts with atmospheric oxygen and catches fire. If it is kept in kerosene it does not react with kerosene and sinks in it. In kerosene, it does not come in contact with oxygen and prevent accidental fire.
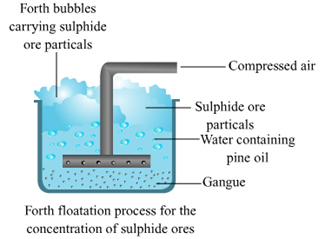
d. Pine oil is used in froth flotation.
Pine oil is added in the froth flotation method to create froth or bubble so that metal can be purify easily because pine oil prevents the ore from gangue for further mixing. Pine oil also acts as best substance for forming froth for the minerals. It also increases the non wettability of mineral particles.
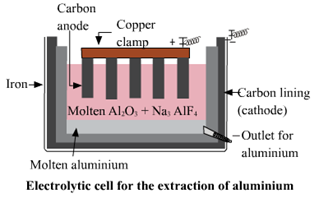
e. Anodes need to be replaced from time to time during the electrolysis of alumina.
In the electrolysis of alumina, graphite rod is used as anode. Hence, the graphite rods i.e. anodes needs to be replaced from time to time during the electrolysis of fused alumina.
Question 6:
When a copper coin is dipped in silver nitrate solution, a glitter appears on the coin after some time. Why does this happen? Write the chemical equation.
Copper is more reactive than silver. Hence, displacement reaction occurs. When copper coin is dipped in silver nitrate solution, it forms copper nitrate and silver metal. A shining white deposit of silver metal is formed on copper coin. As a result shiny coat of silver is formed on the coin. Chemical equation:
2AgNO3(aq) + Cu(s) → Cu(NO3)2(aq) + 2Ag(s)
Question 7:
The electronic configuration of metal ‘A’ is 2,8,1 and that of metal ‘B’ is 2,8,2. Which of the two metals is more reactive? Write their reaction with dilute hydrochloric acid.
If the number of electrons in the outer most orbit less, then the metal is more reactive. Reaction with dil. hydrochloric acid: 2Na + 2HCl → 2NaCl + H2 Mg + 2HCl → MgCl2 + H2
Question 8:
Draw a neat labelled diagram.
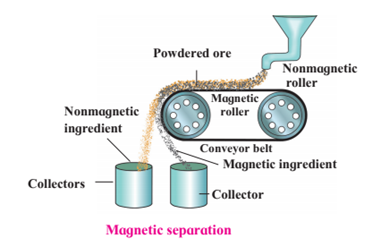
1. Magnetic separation method.
Magnetic separation method.
2. Froth floatation method.
Froth floatation method.
3. Electrolytic reduction of alumina.
Electrolytic reduction of alumina.
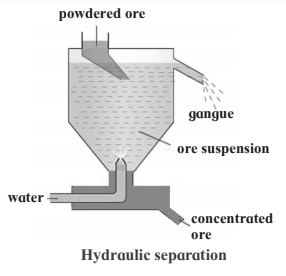
4. Hydraulic separation method.
Hydraulic separation method.
Question 9:
Write chemical equation for the following events.
1. Aluminium came in contact with air.
When Aluminium came in contact with air, it develops a thin layer of aluminium oxide.
Chemical Equation:
4Al + 3O2 \(\underrightarrow{heat}\) 2Al2O3
2. Iron filings are dropped in aqueous solution of copper sulphate.
Iron is more reactive than copper. When iron fillings are dropped in copper sulphate solution, iron displaces Copper (Cu) from its salt Copper sulphate (CuSO4) solution the iron fillings get coated with reddish brown copper metal and the blue color of copper sulphate fades gradually and ferrous sulphate is formed. \(Fe +\frac{CuSO_4}{Blue} → \frac{FeSO4}{colorless} +\frac{Cu}{copper}\)
Chemical Equation:
3. A reaction was brought about between ferric oxide and aluminium.
Aluminium is more reactive than iron. Aluminium metal replaces iron from ferric oxide to form aluminium oxide and iron. This reaction is highly exothermic and it is known as thermit reaction
Chemical Equation:
2Al + Fe2O3 → Al2O3 + 2Fe + Heat
4. Electrolysis of alumina is done.
Ionization of Alumina: Al2O3 → 3O-2 + 2Al+3
Reaction at Cathode: Al+3 + 3e- → Al +4e- (Reduction)
Reaction at Anode: 2O- + 3e-→ O2 + 4e- (Oxidation), C + O2 → CO2
5. Zinc oxide is dissolved in dilute hydrochloric acid.
Zinc Oxide is an inorganic compound with formula ZnO. It is insoluble in water. When Zinc oxide reacts with hydrochloric acid it forms zinc chloride and water.
Chemical Equation:
ZnO(s) + HCl(l) → ZnCl2(aq) + H2O(l)
Question 10:
Complete the following statement using every given options.
During the extraction of aluminium..............
a. Ingredients and gangue in bauxite.
b. Use of leaching during the concentration of ore.
c. Chemical reaction of transformation of bauxite into alumina by Hall’s process.
d. Heating the aluminium ore with concentrated caustic soda.
a. Bauxite is the main ore of aluminium. Silica (SiO2), ferric oxide (Fe2O3) and titanium oxide (TiO2) are the impurities present in bauxite. b. The separation of impurities(silica (SiO2), ferric oxide (Fe2O3) and titanium oxide (TiO2)) in bauxite ore is done by leaching process using either Bayer’s method or Hall’s method. c. In the Hall’s process the bauxite is in powdered form and then leached by heating with aqueous sodium carbonate in the digester to form water soluble sodium aluminate. Then the insoluble impurities are filtered out. The filtrate is warmed and neutralised by passing carbon dioxide gas through it. This results in the precipitation of aluminium hydroxide. 2NaAlO2(aq) + 3H2O + CO2(g)→ 2Al(OH)3 + Na2CO3 The precipitate of Al(OH)3 obtained in both, Bayer’s and Hall’s processes is filtered, washed, dried and then calcined by heating at 10000C to obtain alumina. d. When aluminium ore is heated with caustic soda (NaOH) solution under high pressure for 2-8 hours at 1400-1500C, water soluble sodium aluminate is formed.
Ingredient in bauxite are molten cryolite (Na3AIF6), fluorspar (CaF2).
Al2O32H2O(s)+ Na2CO3(aq) →2NaAlO2(aq) + CO2 + 2H2O(l)
2Al(OH)3 → Al2O3 + 3H2O
Al2O3+ 2NaOH→ 2NaAlO2 + H2O
Question 11:
Divide the metals Cu, Zn, Ca, Mg, Fe, Na, Li into three groups, namely reactive metals, moderately reactive metals and less reactive metals.
Highly reactive metals
moderately reactive metals
less reactive metals.
Ca
Zn
Cu
Mg
Fe
Na
Li
Useful links :
| Main Page : - Maharashtra Board Class 10 Science & Technology Part-1,Part-2 - All chapters notes, solutions, videos, test, pdf.
Books : MSBSHSE -Class 10th Science Text Books – Chapter wise Text Book PDF of all chapter for download Videos : MSBSHSE Class 10th Science & Technology-1-Videos - Chapter wise Videos of all chapter. Previous Chapter : Chapter-7-Lenses - Online Solution Next Chapter : Chapter 9- Carbon compounds -Online Solution |