Acids, Bases and Salts
Maharashtra Board-Class 9-Science & Technology-Chapter-5
Solution
Question 1:
Identify the odd one out and justify.
(a) Chloride, nitrate, hydride, ammonium
Ammonium - Ammonium is odd because Ammonium is cation and rest are anions.
(b) Hydrogen chloride, sodium hydroxide, calcium oxide, ammonia
Hydrogen chloride is odd because Hydrogen chloride is acid and rest are base.
(c) Acetic acid, carbonic acid, hydrochloric acid, nitric acid
Acetic acid is odd because Acetic acid is organic acid, others are inorganic acid
(d) Ammonium chloride, sodium chloride, potassium nitrate, sodium sulphate.
Ammonium chloride is odd because it is acidic salt and rest all are neutral salts.
(e) Sodium nitrate, sodium carbonate, sodium sulphate, sodium chloride.
Sodium carbonate is odd because solution of sodium carbonate is basic, others are neutral salt.
(f) Calcium oxide, magnesium oxide, zinc oxide, sodium oxide.
Zinc oxide is odd because it is amphoteric in nature and other ions are basic in nature
(g) Crystalline blue vitriol, crystalline common salt, crystalline ferrous sulphate, crystalline sodium carbonate.
Crystalline Common salt is odd because on heating, there is no change in color of compound. But in rest of the compounds, there is change in colour, they are crystalline substances contain water of crystallization.
(h) Sodium chloride, potassium hydroxide, acetic acid, sodium acetate.
Acetic acid is odd one because acetic acid is weak electrolytes others are strong electrolytes.
Question 2:
Write down the changes that will be seen in each instance and explain the reason behind it.
(a) 50ml water is added to 50ml solution of copper sulphate.
When 5o mL water is added to 50 mL solution of copper sulphate, then reversible reaction occurs and the colour change from pale blue to white. Also the concentration of copper sulphate solution decreases.
(b) Two drops of the indicator phenolphthalein were added to 10ml solution of sodium hydroxide.
(c) Two or three filings of copper were added to 10ml dilute nitric acid and stirred.
When two to three fillings of copper were added to 10ml dilute nitric acid and stirred, it forms copper nitrite and hydrogen gas.
(d) A litmus paper was dropped into 2ml dilute HCl. Then 2ml concentrated NaOH was added to it and stirred.
When litmus paper is dipped into 2 mL of dilute HCl solution , then blue litmus paper is turned into red colour and there is no effect on red litmus paper. Again, if the 2 mL of concentrated NaOH solution added in the same solution, same litmus paper is dipped into, then red litmus paper turns into blue colour but there is no effect on blue litmus paper. This is due to the respective properties of blue and red litmus paper with acid and base.
(e) Magnesium oxide was added to dilute HCl and magnesium oxide was a added to dilute NaOH.
(f) Zinc oxide was added to dilute HCl and zinc oxide was added to dilute NaOH.
When Zinc oxide is added to dilute HCl, it forms zinc chloride and water and neutralization reaction takes place. In this reaction zinc oxide is basic oxide When zinc oxide was added to dilute NaOH. Zinc oxide reacts with sodium hydroxide and it forms zincate sodium and water. In this reaction zinc oxide is acidic oxide ZnO + 2NaOH→Na2ZnO2 + H2O Therefore zinc oxide is an amphoteric oxide because it shows both acidic and basic properties.
ZnO +2HCl → ZnCl2 + H2O
(g) Dilute HCl was added to limestone.
Limestone is calcium carbonate. When Dilute HCl was added to limestone, it forms calcium chloride, water and carbon dioxide gas. 2HCl + CaCO3 → CaCl2 + CO2 +H2O
(h) Pieces of blue vitriol were heated in a test tube. On cooling, water was added to it.
(i) Dilute H2SO4 was taken in an electrolytic cell and electric current was passed through it.
When a dilute solution of sulfuric acid is electrolysed, gases are produced at both the anode and the cathode electrode. The gas produced at the cathode burns with a 'pop' sound, when a sample is lit with a lighted splint. This shows that the gas is hydrogen. 2H+ +2e- → H2 The gas produced at the anode relights a glowing splint dipped into a sample of the gas. This shows that the gas is oxygen. 4OH- - 4e- → 2H2O + O2 The gases are produced when ions move towards the electrodes.
Question 3:
Classify the following oxides into three types and name the types.
CaO, MgO, CO2 , SO3 , Na2O, ZnO, Al2O3 , Fe2O3
1) Acidic Oxides: CO2 (Carbon dioxide), SO3 (Sulfur trioxide) 2) Basic Oxides: CaO (Calcium oxide), MgO (Magnesium oxide), Na2O (Sodium oxide), Fe2O3 (Ferric oxide) 3) Amphoteric Oxides: ZnO (Zinc oxide), Al2O3 (Aluminium oxide),
Question 4:
Explain by drawing a figure of the electronic configuration.
1. Formation of sodium chloride from sodium and chlorine.
Atomic number of Sodium (Na) atom is 11. Atomic number of Chlorine(Cl) atom is 17.
Electronic configuration is:
Na = 2, 8, 1
So it contains 1 valence electron. In order to achieve the nearest noble gas configuration, it loses one electron to form Sodium ion.
Na+ = 2,8
Electronic configuration is:
Cl = 2, 8, 7
So it contains 7 valence electron. In order to achieve the nearest noble gas configuration, it gains one electron to form Chloride ion.
Cl- = 2,8,8
An Ionic bond is formed between sodium ion and chloride ion by complete transfer of electron from sodium to chlorine, due to electrostatic force of attraction, giving rise to ionic compound sodium chloride.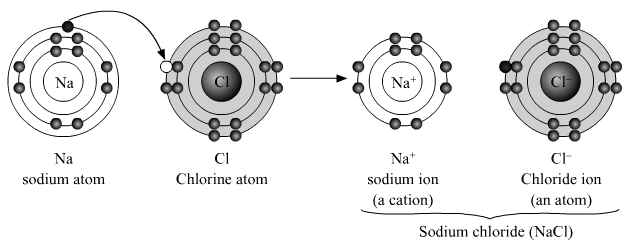
2. Formation of a magnesium chloride from magnesium and chlorine.
Atomic number of Magnesium atom is 12. Atomic number of Chlorine(Cl) atom is 17.
Electronic configuration is :
Mg = 2, 8, 2
So it contains 2 valence electron. In order to achieve the nearest noble gas configuration, it loses two electrons to form Magnesium ion.
Mg2+ = 2,8
Electronic configuration is :
Cl = 2, 8, 7
So it contains 7 valence electron. In order to achieve the nearest noble gas configuration, it gains one electron to form Chloride ion.
Cl- = 2,8,8
Due to the electrostatic force of attraction an Ionic bond is formed between Magnesium ion and two Chloride ion by complete transfer of one electron to each Chlorine ion and this results in the formation of magnesium chloride.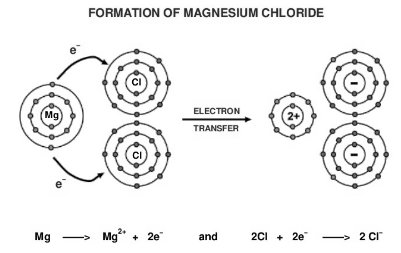
Question 5:
Show the dissociation of the following compounds on dissolving in water, with the help of chemical equation and write whether the proportion of dissociation is small or large.
Hydrochloric acid, Sodium chloride, Potassium hydroxide, Ammonia, Acetic acid, Magnesium chloride, Copper sulphate
(i) HCl \(\underrightarrow{water}\) H+ + Cl- Dissociation is large (ii) NaCl \(\underrightarrow{water}\) Na+ + Cl- Dissociation is large (iii) KOH \(\underrightarrow{water}\) K+ + OH- Dissociation is large (iv) NH3 \(\underrightarrow{water}\) NH4+ + OH- Dissociation is small (v) CH3COOH \(\underrightarrow{water}\) CH3COO- + H+ Dissociation is small (vi) MgCl2 \(\underrightarrow{water}\) Mg2+ + Cl- Dissociation is small (vii) CuSO4 \(\underrightarrow{water}\) Cu+2 + SO4-2 Dissociation is large
Question 6:
Write down the concentration of each of the following solutions in g/L and mol/L.
1) 7.3g HCl in 100ml solution
1) 7.3 g HCl in 100 mL solution: Molecular Formula = HCL Molecular mass of HCl = 1+35.5=36.5 m Mass of HCL in Mole (mol) = Mass of HCL in gram(g)/ Molecular mass(m) = 7.3/36.5 =0.2 mol Concentration of HCl (g/L) =Mass of solute (g)/Volume(L) = 7.3g/0.1L = 73 g/L Concentration of HCL (mol/L) =Mass of HCL in moles/Volume of HCl in L = 0.2mol/0.1L = 2 mol/L Answer is 73 g/L & 2 mol/L
Given: Volume of solution = 100ml =0.1L, Mass of Solute in gram =7.3g
2) 2g NaOH in 50 mL solution Given: Volume of solution = 50ml =0.05L, Mass of NaOH in gram =2g Molecular Formula = NaOH Molecular mass of NaOH = 23 +16 + 1 = 40m Mass of HCL in Mole (mol) = Mass of NaOH in gram(g)/ Molecular mass(m) = 2/40 =0.05 mol Concentration of NaOH (g/L) =Mass of solute (g)/Volume(L) = 2g/0.05L = 40 g/L Concentration of NaOH (mol/L) =Mass of NaOH in moles/Volume of NaOH in L = 0.05mol/0.05L = 1 mol/L Answer is 40 g/L & 1 mol/L
3) 3g CH3COOH in 100 mL solution Given: Volume of solution = 100ml =0.1L, Mass of CH3COOH in gram =3g Molecular Formula = CH3COOH Molecular mass of CH3COOH = 2(C)+2(O)+4(H) =2 × 12 + 2 × 16 + 4 × 1 = 60m Mass of CH3COOH in Mole (mol) = Mass of CH3COOH in gram(g)/ Molecular mass(m) = 3/60 = 0.05 mol Concentration of CH3COOH (g/L) =Mass of solute (g)/Volume(L) = 3g/0.1L = 30 g/L Concentration of CH3COOH (mol/L) = Mass of CH3COOH(mol)/Volume of CH3COOH (L) =0.05mol/0.1L = 0.5 mol/L Answer is 30 g/L & 0.5 mol/L
4) 4.9 g H2SO4 in 200 mL solution Given: Volume of solution = 200ml =0.2L, Mass of CH3COOH in gram =4.9g Molecular Formula = H2SO4 Molecular mass of H2SO4 = 2(H)+1(S)+4(O) = 2 × 1 + 32 + 4 ×16 = 98m Mass of H2SO4 in Mole (mol) = Mass of H2SO4 in gram(g)/ Molecular mass(m)= 4.9/98 = 0.05 mol Concentration of H2SO4 (g/L) =Mass of solute (g)/Volume(L) = 4.9g/0.2L = 24.5 g/L Concentration of H2SO4 (mol/L) = Mass of H2SO4 (mol)/Volume of H2SO4 (L) = 0.05mol/0.2L = 0.25 mol/L Answer is 24.5 g/L & 0.25 mol/L
Question 7:
Obtain a sample of rainwater. Add to it a few drops of universal indicator. Measure its pH. Describe the nature of the sample of rainwater and explain the effect if it has on the living world.
This is an activity based question in which you are supposed to collect rainwater from different places. If we take samples of rain water from different places, we observe the following results :
Question 8:
Answer the following questions.
1. Classify the acids according to their basicity and give one example of each type.
The number of ionizable hydrogen (H+) ions obtainable by dissociation of one molecule of an acid is called its basicity H2SO4 ------> 2H+ + SO42- Based on Basicity acids were classified into different types: 1. Mono-basic acids: Acids, which on ionization produce one hydrogen ion. Example: HCl, HNO3 etc. 2. Di-basic acids: Acids, which on ionization produce two hydrogen ion Example: H2SO4, H2CO3 etc. 3. Tri-basic acids: Acids, which on ionization produce three hydrogen ion Example: H3PO4, H3PO3 etc.
For example :
HCl ---------> H+ + Cl-
Basicity of HCl is 1.
Basicity of H2SO4 is 2.
1. Mono-basic acids
2. Di-basic acids
3. Tri-basic acids
2. What is meant by neutralization? Give two examples from everyday life of the neutralization reaction.
Neutralization reaction : A neutralization reaction is a reaction in which an acid and a base reacts to form water and a salt. It involves the combination of H+ ions and OH- ions to generate water. Acid + Base → Salt + Water HCL(aq) + NaOH(aq) → NaCl + H2O H+ ions from acid and OH- ions are formed from base H+ (aq) + OH- (aq) → H2O(l) H+ ions from an acid and OH- ions from a base to form unionized water, this is called neutralization. When a solution is neutralized, it means that salts are formed from equal weights of acid and base. Neutralization reaction has application in daily life: 1) Acidity in digestive system : 2) Tooth decay : 3) Plant growth :
An acidic stomach due to eating too much spicy food, can be relieved by taking an antacid. The antacid is alkaline/basic in nature and helps to neutralize the stomach's acidity or you may take magnesium hydroxide(Milk of magnesia) and sodium hydrogen carbonate(Baking soda).
When we eat food containing sugar, then bacteria present in our mouth break down the sugar to form acids(such as lactic acid). Thus acid is formed in the mouth after digestion. This will lead to the cause of tooth decay. The best way to prevent tooth decay is to clean the mouth after eating food with toothpaste, which is basic in nature. This will result in neutralization of acid by base.
Most of the plants grow best when the pH of the soil is close to 7 that's neural. If the soil is too acidic or too basic (alkaline), the plants grow badly.
The acidic soil is neutralize by treatment with materials like quicklime (calcium oxide) or slaked lime (calcium hydroxide) or chalk(calcium carbonate).
If the soil is too basic, then alkalinity can be reduced by adding decaying organic matter (manure or composite) which contains acidic materials.
3. Explain what is meant by electrolysis of water. Write the electrode reactions and explain them.
Electrolysis of water : The following equation represents the electrolysis of water :H2O(l) In pure water, at the negatively charged cathode, a reduction reaction takes place, with electrons (e−) from the cathode being given to hydrogen cations to form hydrogen gas. Reduction at cathode: 2 H+ + 2e− → H2 On positively charged anode, an oxidation reaction occurs, generating oxygen gas by giving electrons to the anode : Oxidation at anode: 2 H2O → O2(g) + 4 H+(aq) + 4e− Overall reaction: 2 H2O(l) → 2 H2(g) + O2(g) The number of hydrogen molecules produced is thus twice the number of oxygen molecules. The produced hydrogen gas has therefore twice the volume of the produced oxygen gas. The number of electrons pushed through the water is twice the number of generated hydrogen molecules and four times the number of generated oxygen molecules.
When electric current is passed through water containing a few drops of an acid or a few drops of a base, it dissociates to give H+ ions and OH-ions. Hydrogen gas is formed near the cathode and oxygen gas near the anode. This is called electrolysis of water.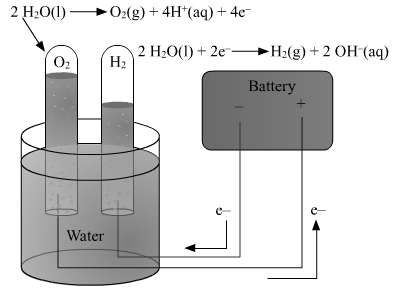
Question 9:
Give reason for the following.
1. Hydronium ions are always in the form H3 O+
The strength of an acid is measured in terms of the extent of the dissociation of acid in an aqueous solution. The higher H+ ion concentration the stronger is the acid. H+ ion does not exist alone H+ ion is being unstable, When an acids dissolve in water. The H+ ion from acid always goes to the nearest water molecule to form hydronium ion. H2O + H+ → H3O+ ion
HCl(aq)+H2O(aq)→H3O+(aq)+Cl-(aq)
2. Buttermilk spoils if kept in a copper or brass container.
Question 10:
Write the chemical equations for the following activities.
(i) NaOH solution was added to HCl solution.
It forms sodium chloride and water is neutralization reaction. HCl(l) + NaOH(l) → NaCl(s) + H2O(l) + Energy
(ii) Zinc dust was added to dilute H2 SO4 .
It forms zinc sulphate and hydrogen gas Zn(s)+ H2SO4(l)→ ZnSO4(s) +H2 (g)
(iii) Dilute nitric acid was added to calcium oxide.
It forms calcium nitrate and water CaO(s) + 2HNO3(l)→ Ca(NO3)2 (s)+ H2O(l)
(iv) Carbon dioxide gas was passed through KOH solution.
It forms potassium carbonate and water KOH(l) + CO2(g)→ KHCO3
(v) Dilute HCl was poured on baking soda.
It forms sodium chloride and carbon dioxide gas. NaHCO3(s)+ 2HCl(l)→ NaCl(s) + H2O (l)+ CO2
Question 11:
State the differences.
1. Acids and bases
Example: HCL, H2SO4 Example :NaOH, Ca(OH)2
Acid
Base
Substance which when dissolved in water gives hydrogen ion
Substance which when dissolved in water can accept hydrogen ions
Substance which donates a proton
Substance which accepts a proton
Strength depends on the concentration of the hydronium ions
Strength depends on the concentration of the hydroxide ions
Acids have a sour taste
Basehave a bitter taste
Releases hydrogen ions (H+) when mixed with water
Releases hydroxide ions(OH-) when mixed with water
pH value less than 7.0
pH value greater than 7.0
Blue litmus paper turns red in acid
Red litmus paper turns blue in base
It neutralizes a base to give salt and water
It neutralizes an acid to give salt and water
2. Cation and anion
Examples : Na+, Mg2+,Ca2+ etc. Examples :Cl-, Br-,S2- etc.
Cations
Anions
Positively charged particles
Negatively charged particles
Formed by loss of electrons from metals
Formed by gain of electrons from non-metals
During electrolysis, it moves towards cathode
During electrolysis, it moves towards anode
size of cation is smaller than its parent atom
size of anion is same or larger than its parent atom
Usually obtain from metals and hydrogen.
Usually obtain from non metals
3. Negative electrode and positive electrode.
Negative Electrode
Positive Electrode
Refers to a piece of electrochemical cell that is the negative pole
Refers to a piece of electrochemical cell that is the positive pole
Connects to negative terminal of a battery by means of wire
Connects to positive terminal of a battery by means of wire
Also called as cathode
Also called as anode
Positive charged cations moves toward it
Negative charged anions moves toward it
accepts electrons to deposited
donates electron
Question 12:
Classify aqueous solutions of the following substances according to their pH into three groups : 7, more than 7, less than 7.
Common salt, sodium acetate, hydrochloric acid, carbon dioxide, potassium bromide, calcium hydoxide, ammonium chloride, vinegar, sodium carbonate, ammonia, sulphur dioxide.
Substances
pH Value
Common salt
equal to 7
Sodium acetate
greater than 7
Hydrochloric acid
less than 7
Carbon dioxide
less than 7
Potassium bromide
equal to 7
Calcium hydroxide
greater than 7
Ammonium chloride
less than 7
Vinegar
less than 7
Sodium carbonate
greater than 7
Ammonia
greater than 7
Sulphur dioxide
less than 7
Useful links :
| Main Page : - Maharashtra Board Class 9th Science & Technology - All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter 4: Measurements of Matter - online Solution Next Chapter : Chapter 6: Classification of plants -online Solution |