Chapter-9-Carbon Compound
Class 10-Science & Technology Part-1-Chapter-9-Maharashtra Board
Solution
Question 1:
Match the pairs.
| Group 'A' | Group 'B' |
| a. C2H6 | 1. Unsaturated hydrocarbon |
| b. C2H2 | 2. Molecular formula of an alcohol |
| c. CH4O | 3. Saturated hydrocarbon |
| d. C3H6 | 4. Triple bond |
Group 'A'
Group 'B'
a. C2H6
3. Saturated hydrocarbon
b. C2H2
4. Triple bond
c. CH4O
2. Molecular formula of an alcohol
d. C3H6
1. Unsaturated hydrocarbon
Question 2:
Draw an electron dot structure of the following molecules. (Without showing the circles)
a. Methane b. Ethene, c. Methanol d. Water
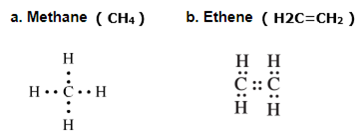
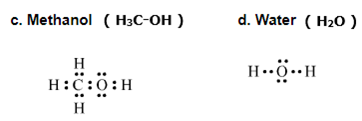
Question 3:
Draw all possible structural formulae of compounds from their molecular formula given below.
(i) C3H8 (ii) C4H10 (iii) C3H4
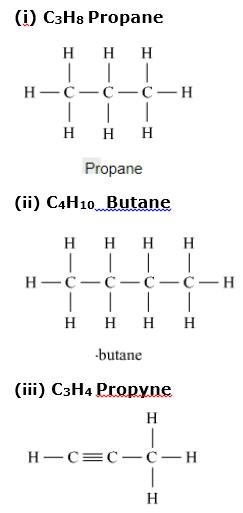
Question 4:
Explain the following terms with example.
1. Structural isomerism
The phenomenon in which compounds having different structural formulae have the same molecular formula is called structural isomerism.
Butane is represented by two different compounds as their structural formulae are different. These two different structural formulae have the same molecular formula i.e. C4H10 Butane
2. Covalent bond
The chemical bond formed by sharing of two valence electron between the two atoms is called covalent bond. It is also known as molecular bond. For example: Molecules that have covalent linkages are hydrogen H2, nitrogen N2, chlorine Cl2, water H2O, and ammonia NH3. A single line indicates a single bond between two atoms (i.e. involving one electron pair), double lines (=) indicate a double bond between two atoms (i.e. involving two electron pairs), and triple lines (≡) represent a triple bond (C≡O). Example : 1) Hydrogen molecule is formed by covalent bonding. Atomic number of hydrogen is 1, its atom contains 1 electron in K shell. It requires one more electron to complete the K shell and attain the configuration of helium (He). To meet this requirement two hydrogen atoms share their electrons with each other to form H2 molecule. One covalent bond, that is a single bond is formed between two hydrogen atoms by sharing of two electrons. 2) The atomic number of oxygen is 8. The electronic configuration is (2,6) Oxygen has 6 electrons in the outermost shell. It requires 2 electrons to complete the L shell and attain the configuration of neon(Ne) The O2 molecule is formed by chemical combination of two oxygen atoms; On drawing the electron-dot structures of these two molecules, it becomes clear that the two oxygen atoms in O2 molecule are joined with each other by two covalent bonds, that is, a double bond.
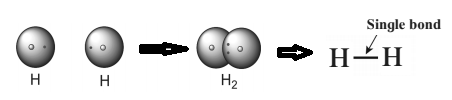
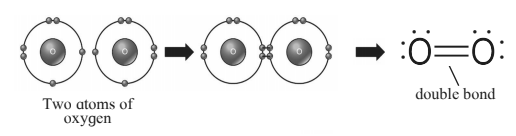
3. Hetero atom in a carbon compound
Hetero atom in a carbon compound: It is a compound formed by replacement of carbon and hydrogen by hetero atom in a compound. A hetero atom is any atom other than carbon or hydrogen. The atoms of these compounds substitute one or more hydrogen atoms in the hydrocarbon chain. Typical heteroatoms are nitrogen, oxygen, sulfur, phosphorus, chlorine, bromine, and iodine. 1-Oxygen is hetero atom in ethanol (C2H5-O-H)
Example:
2-Nitrogen is the hetero atom in Ethyl amine ( CH3-CH2- NH2)
4. Functional group
The compound acquire specific chemical properties due to these hetero atoms or the groups of atoms that contain hetero atoms, irrespective of length and nature of the carbon chain in that
compound. Therefore these hetero atoms or groups of atoms containing hetero atoms are called the functional groups.
Example: Methyl alcohol, acetic acid.
5. Alkane
Alkane: In hydrocarbon, the four valancies of carbon atoms are satisfied only by the single bonds such compounds are called alkane. Alkane is a saturated hydrocarbon. It is formed, when there is sharing of one electron pair between carbon atoms in a compound. In which the carbon atoms are linked to each other by single bonds. The general formula for alkane is CNH2N+2, where N is equal to no of carbon atom in a compound. They are chemically less reactive.
For example:
C2H6
Ethane
C3H8
propane
C4H10
butane
C5H12
pentane
C6H14
hexane
C7H16
heptane
6. Unsaturated hydrocarbon
Unsaturated hydrocarbon: An unsaturated hydrocarbon is a hydrocarbon containing at least one double or triple bond. They are chemically more reactive.
Example:
Alkenes - These unsaturated hydrocarbons are molecules that contain at least one carbon-to-carbon double bond. With the chemical formula consisting of CnH2n. The simplest alkene is ethylene.
Alkynes - These unsaturated hydrocarbons are molecules that contain at least one carbon-to-carbon triple bond. Acetylenes are common examples of alkynes.
7. Homopolymer
Homopolymer: A homopolymer is a polymer formed from same type of monomer units.
For examples: Polyvinylchloride (PVC), Polyethylene(-CH2- CH2-)n, Polystyrene are homopolymer.
8. Monomer
Monomer: A monomer is a molecule that forms the basic unit for polymers. They may be considered as building blocks from which proteins are made. Monomers may bind to other monomer unit to form a repeating chain molecule. Monomers may be either natural or synthetic in origin.
For example: Ethylene, vinyl chloride, styrene etc.
9. Reduction
Reduction: The addition of hydrogen to a substance is called reduction. The removal of oxygen from a substance is called reduction.
For example:
2Ag2O → 4 Ag + O2 ↑
In a reaction, silver oxide is changing to silver. That is, oxygen is being removed from silver oxide. Removal of oxygen from substance is called reduction, so silver oxide undergoes reduction.
NiO + H2 → Ni + H2O
In a reaction, Nickle oxide is changing to nickle. That is, oxygen is being removed from nickle oxide. Removal of oxygen from substance is called reduction, so nickle oxide undergoes reduction.In a reaction, hydrogen is changing to H2O. That is,oxygen is being added to hydrogen. Addition of oxygen to a substance is called oxidation, so hydrogen undergoes oxidation.
10. Oxidant
Oxidant:
The substance which gives oxygen for oxidation is called an oxidising agent or oxidant.
The substance which removes hydrogen is called an oxidising agent or oxidant.
For example:
CuO + H2 → Cu + H2O
Oxidising agent=CuO
Reducing agent= H2
Substance oxidised=H2
Substance reduced=CuO
Question 5:
Write the IUPAC names of the following structural formulae.
1. CH3-CH2-CH2-CH3
The number of items in the longest chain :4 Parent Alkane :Butane IUPAC Name :nButane
2. CH3-CHOH-CH3
The number of items in the longest chain :3 Parent Alkane :Propane IUPAC Name : Propan-2-ol
3. CH3-CH2-COOH
The number of items in the longest chain :3 Parent Alkane :Propane IUPAC Name : Propanoic acid
4. CH3-CH2-NH2
The number of items in the longest chain :2 Parent Alkane : Ethane IUPAC Name : Ethanamine
5. CH3-CHO
The number of items in the longest chain :2 Parent Alkane : Ethane IUPAC Name : Ethanal
6. CH3-CO-CH2-CH3
The number of items in the longest chain :4 Parent Alkane : Butane IUPAC Name : Butan-2-one
Identify the type of the following reaction of carbon compounds.
1. CH3 -CH2 -CH2-OH → CH3 -CH2 -COOH
2. CH3 -CH2 -CH3 → 3CO2 + 4H2O
3. CH3 -CH = CH -CH3 + Br2 → CH3 -CHBr - CHBr -CH3
4. CH3 -CH3 + Cl2 → CH3 -CH2 -Cl + HCl
5. CH3 -CH2 -CH2 -CH2 -OH → CH3 -CH2 -CH=CH2 + H2O
6. CH3 -CH2 -COOH + NaOH → CH3 -CH2 -COO - Na+ + H2O
7. CH3 -COOH + CH3 -OH → CH3 -COO- CH3 + H2O
1. CH3-CH2-CH2-OH → CH3 -CH2 -COOH = Oxidation reaction(acidic KMnO4) 2. CH3 -CH2 -CH3 → 3CO2 + 4H2O = Combusion reaction 3. CH3 -CH = CH -CH3 + Br2 → CH3 -CHBr - CHBr -CH3 = Addition reaction 4. CH3 -CH3 + Cl2 → CH3 -CH2 -Cl + HCl = Substitution reaction 5. CH3 -CH2 -CH2 -CH2 -OH → CH3 -CH2 -CH=CH2 + H2O = Dehydration reaction 6. CH3 -CH2 -COOH + NaOH → CH3 -CH2 -COO - Na+ + H2O = Neutralization reaction(reaction with base) 7. CH3 -COOH + CH3 -OH → CH3 -COO- CH3 + H2O = Esterification reaction
Question 7:
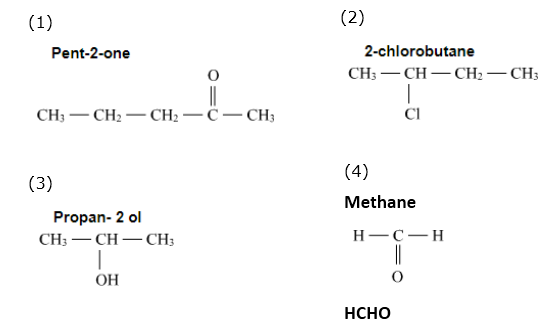
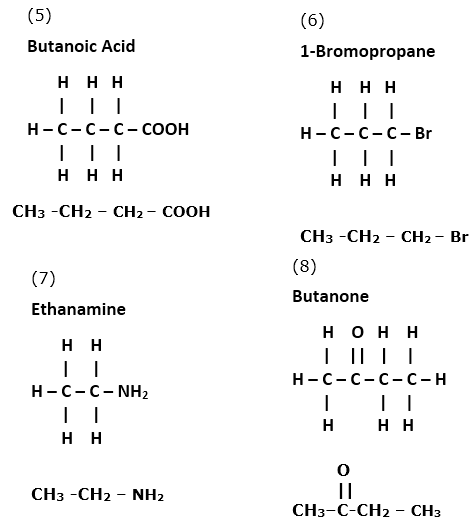
Write structural formulae for the following IUPAC names.
| 1. pent-2-one | 2. 2-chlorobutane |
| 3. propan- 2 ol | 4. methanal |
| 5. butanoic acid | 6. 1-bromopropane |
| 7. ethanamine | 8. butanone |
Question 8:
Write answers as directed.
a. What causes the existence of very large number of carbon compound ?
(1) Carbon has a unique ability to form strong covalent bonds with other carbon atoms, this results in formation of big molecules. This property of carbon is called catenation power. The carbon. has 4 valence electrons. That means each individual carbon atom can bind to 4 other atoms of almost any variety and each of those 4 can bind to 4 others atoms. This leads to formation of organic compounds having incredible variety and complexity - short chains, long chains, ring structures, branched structures and so on. (2) One, two or three covalent bonds can bond together two carbon atoms. These bonds are called single covalent bond, double covalent bond and triple covalent bond respectively. Due to the ability of carbon atoms to form multiple bonds as well as single bonds, the number of carbon compounds increases. (3) Carbon being tetravalent, one carbon atom can form bonds with four other atoms (carbon or any other). This results in formation of many compounds. (4) Isomerism is one more characteristie of carbon compound which is responsible for large This characteristics leads to existance of very large number of carbon compound.
number of carbon compounds.
b. Saturated hydrocarbons are classified into three types. Write these names giving one example each.
Saturated hydrocarbons are classified into three types:
Saturated hydrocarbon
Example
Structure
1) Straight chain hydrocarbons
Propane C3H8
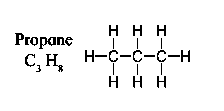
2)Branched chain hydrocarbon
isobutane C4H10
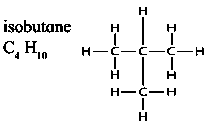
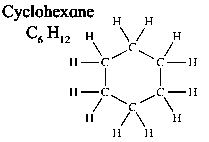
3)Cyclic hydrocarbon
Cyclohexane C6H12
c. Give any four functional groups containing oxygen as the heteroatom in it. Write name and structural formula of one example each.
Four functional groups containing oxygen as the heteroatom in it are as follows: – O – H Condensed Structural Formula : – OH Example: Methanol 2. Aldehydes: The functional group, which is present in an aldehyde, is -CHO. The IUPAC group suffix of an aldehyde is –al. Condensed Structural Formula : – CHO Example: Formaldehyde 3. Ketones: The functional group, which is present in a ketone is >C=O. The IUPAC group suffix of a ketone is –one. Condensed Structural Formula : – CO – Example: Acetone 4. Carboxylic acid: The functional group present in a carboxylic acid is -COOH. The IUPAC group suffix of a carboxylic acid is –oic acid. Condensed Structural Formula : – COOH Example: Acetic acid
1. Alcohols: The functional group, which is present in alcohol, is -OH. The IUPAC group suffix of alcohol is –ol.
Structural Formula :
Structural Formula :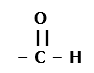
Structural Formula : 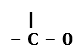
Structural Formula :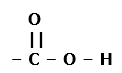
c. Give names of three functional groups containing three different hetero atoms. Write name and structural formula of one example each.
Three functional groups containing three different hetero atoms are as follows: Condensed Structural Formula : – COOH Example: Acetic acid Amine: The functional groups present in an amine are -NH2 Here, heteroatom is nitrogen. Structural Formula : Condensed Structural Formula : – NH2 Example: Methylamine Halides: The functional group presence in halides is X(halogen= F,Cl,Br,I).Here, heteroatom is chloride. Structural Formula : – O – H Condensed Structural Formula : – OH Example: Chloromethane
Carboxylic acid: The functional group present in a carboxylic acid is -COOH. Here, heteroatom is oxygen.
Structural Formula :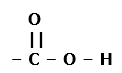
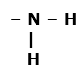
e. Give names of three natural polymers. Write the place of their occurance and names of monomers from which they are formed.
Natural polymers
Monomer unit
Occurance
1.Polysaccaride
Glucose
Starch
2.Cellulose
Glucose
Wood (cell wall of plant cells)
3.Proteins
alpha aminoacids
Muscles, Hairs, Skin, Egg
4.Rubber
Isoprene(CH2=C(CH3)-CH=CH2 )
Latex of rubber tree
f. What is meant by vinegar and gashol? What are their uses ?
Vinegar is 5-8% solution of acetic acid ( Ethanoic acid ). It is basically produce by the process of fermentation of ethanol through ethanoic acid in the presence of bacteria . Uses of Vinegar are: Gasohol is a mixture of 90% gasoline and 10% of anhydrous Alcohol (Ethyl Alcohol). It is commonly known as the alternative fuel or a motor fuel. Benefits of gasohol are :- Uses of gasohol are:
g. What is a catalyst ? Write any one reaction which is brought about by use of catalyst ?
Catalyst is a substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change. Example:
E.g.
Name of process
Equations (in words or formulae)
Catalyst
1
Fermentation of glucose to form ethanol
Glucose Ethanol + Carbon dioxide
Specific enzymes (in yeast)
2
Hydration of ethene to form ethanol
Ethene + Water (Steam) Heat Ethanol
Phosphoric Acid
3
Hydrogenation of unsaturated fats
(to harden oils in the manufacture of margarine)Hydrogen + Unsaturated Fats Saturated Fats
Nickel (Ni)
4
Haber's process
Nitrogen +Hydrogen Ammonia
Iron
| View Notes of this Chapter ← Coming Soon.... |
Videos
Click on below link to Play Video
1- Introduction
2- What is special about carbon
8- Catenation
12- Electron dot structure hydrocarbon
13- Electron dot structure Examples
14- Alkyl group
16- IUPAC for branched hydrocarbon
17- Isomers
21- Examples
24- Coal & Petroleum
25- Ethanol
26- Ethanoic Acid
27- Soap
28- Detergent
29- Symmary
Click on below link to Download PDF from store
Class 10-Science & Technology-1-Chapter-9-Carbon Compounds -Notes
Class 10-Science & Technology-1-Chapter-9-Carbon Compounds - Solutions
Class 10-Science & Technology-1-Chapter-9-Carbon Compounds - Books
Useful links :
| Main Page : - Maharashtra Board Class 10 Science & Technology Part-1,Part-2 - All chapters notes, solutions, videos, test, pdf.
Books : MSBSHSE -Class 10th Science Text Books – Chapter wise Text Book PDF of all chapter for download Videos : MSBSHSE Class 10th Science & Technology-1-Videos - Chapter wise Videos of all chapter. Previous Chapter : Chapter-8-Metallurgy - Online Solution Next Chapter : Chapter 10. Space Missions - Coming Soon |
I loved the format of answers but I think it can be done better. It will help students for better understanding.😀😀