Measurement and Effect of Heat
Maharashtra Board Class 8- General Science - Chapter-14
Notes
|
Topics to be learn :
|
Introduction : We get heat from
- Sun : The Sun is the biggest source of heat received by the earth. A large amount of heat is generated due to the nuclear fusion taking place in its centre.
- Earth : As the temperature at the centre of the earth is high, the earth is also a source of heat. This heat is called geothermal energy
- Chemical energy : Fuels like wood, coal, petrol, when burns there is chemical reaction between the fuel and oxygen. Heat is generated in these reactions.
- Electricity : Several equipments which produce heat with the help of electricity e.g. electric press, electric heater etc.
- Atomic energy : A huge amount of heat is produced in a very short time when the nuclei of some elements like uranium, thorium etc undergo fission.
- Air : A large amount of heat is present in the air around us
Energy : The energy stored in a body because of its specific state or position is called its potential energy. The energy possessed by a body because of its motion is called its kinetic energy.
Heat : It is a form of energy. It flows from a body at higher temperature to a body at lower temperature. The total kinetic energy of the atoms in a substance is a measure of the heat contained in that substance.
Heat is transferred by conduction, convection and radiation.
Effects of heat : Expansion, change of state, rise in temperature, emission of light, burning.
The SI unit of heat is the joule. Heat, can be expressed in erg also. Heat is also expressed in calorie and kilocalorie.

Temperature : It is a quantitative measure of the degree of hotness or coldness of a body. It decides the direction of flow of heat. The temperature of a substance is related to the average kinetic energy of the atoms in that substance.
Temperature is measured in units of Celsius (0C), Fahrenheit (0F) and Kelvin (K). Kelvin is used in scientific experiments, while the other two are used in daily life. The relation between the three units is shown by the following formulae.
\(\frac{F-32}{9}=\frac{C}{5}\), K = C + 273.15
∴ F = \(\frac{9}{5}\)C+32 = \(\frac{9}{5}\)(K − 273.15) + 32
Here, C denotes temperature in °C, F denotes temperature in °F and K denotes temperature in K (kelvin).
Here, C denotes temperature in °C, F denotes temperature in °F and K denotes temperature in K (kelvin).
| Description | °F | °C | K |
| Boiling point of water | 212 | 100 | 373.15 |
| Freezing point of water | 32 | 0 | 273 |
| Room temperature | 72 | 23 | 296 |
| Boiling point of mercury | 356.7 | ||
| Freezing point of mercury | -38.8 |
Thermometer : A thermometer is a device to measure temperature.
Types of thermometer as per construction :
- A thermometer containing mercury in its bulb is called a mercury thermometer.
- There are other types of thermometer such as a thermocouple thermometer, a platinum resistance thermometer, a thermistor thermometer, etc.
Types of thermometer as per use :
- There are different types of thermometers such as that used in a laboratory, clinical thermometer, digital thermometer and maximum – minimum thermometer.
Liquid (mercury or alcohol) thermometer : Ans. A thermometer in which the change in the volume of a liquid (mercury or alcohol) with temperature is used for measurement of temperature is called a liquid thermometer.
- Because mercury is harmful for us, it has been replaced by alcohol in a thermometer.
[Notes : Mercury thermometers are still widely used in laboratories in schools and colleges.]
Clinical thermometer : A clinical thermometer has a narrow stem and a long bulb filled with mercury (or alcohol). There is a small constriction in the stem above the bulb.
- When the bulb of the thermometer is held in the armpit or the mouth of a patient, the mercury (or alcohol) in the bulb rises in the stem.
- When it is taken out of the patient’s body, the small constriction does not allow the mercury (or alcohol) from the stem to retreat into the bulb.
- Thus, this arrangement enables us to read the temperature of the patient’s body at ease after the removal of the thermometer from his body.
- The clinical thermometer is graduated from 35°C to 42°C (or from 95°F to 108°F). At 37°C (98.6°F), there is a red arrow mark which indicates the temperature of a healthy person.
- The bulb of the thermometer is long. The stem is thin, hence it increases the sensitivity of the thermometer.
- The surface of the stem is in the form of a magnifying -glass and hence it enables us to see the thin thread of mercury clearly.
- The thermometer used in a laboratory has wider range and does not have constriction like a clinical thermometer.
Specific heat : The specific heat of an object (a substance) is the amount of heat required to increase the temperature of unit mass of that substance through one degree. It is represented by the symbol c and expressed in different units such as J/(kg-K), J/(kg-°C) and cal/ (g-°C).
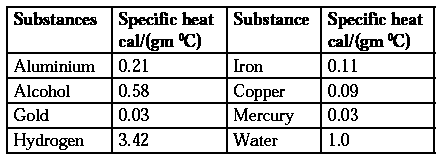
Heat absorbed or given out by a body, Q= m c (Tf - Ti)
Calorimeter : A calorimeter is used to measure the heat content of an object.
The heat produced or absorbed in a physical or chemical process can measure by using calorimeter. The specific heat of a body can be determined using a calorimeter.
Construction of a calorimeter :
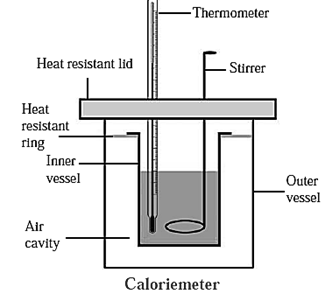
- Like a thermoflask, a calorimeter has two vessels. The inner vessel, made of copper, is (practically) thermally isolated from the surroundings.
- The outer vessel is made of wood and is covered with a heat resistant lid. The lid has two holes, one for the thermometer and the other for the stirrer.
- The inner and outer surfaces of the inner vessel are polished for minimizing exchange of heat with the surroundings by radiation.
- A heat resistant ring covers the inner vessel.
As a calorimeter ensures that there is hardly any exchange of heat between the contents of the calorimeter and the surroundings, the calorimeter is used in the study of the exchange of heat between a solid and liquid or between two liquids.
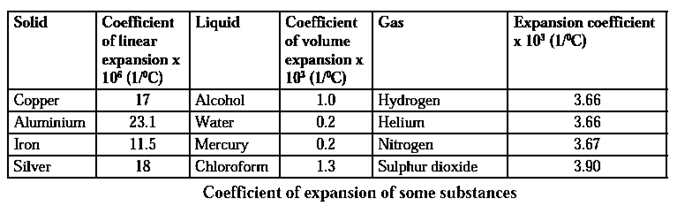
Coefficient of linear expansion of a solid :
Suppose a rod of length l1 at temperature T1 is heated to temperature T2 such that ΔT = T2 - T1 is very small. Let l2 be the length of the rod at temperature T2
Experimentally, it is found that the increase in the length of the rod (linear expansion), l2 — l1, is proportional to l1 and ΔT. Therefore,
(l2 - l1) ∝ (l1 ΔT)
(l2 - l1) =λ (l1 ΔT)
where λ is the constant of proportionality, called the coefficient of linear expansion of the solid.
λ = \(\frac{l_2-l_1}{l_1ΔT}\). It is expressed in per °C
We have
l2 = l1 + λ(l1 ΔT) = l1(1 +λ ΔT).
Coefficient of areal expansion of a solid :
Suppose a sheet of a solid with surface area A1 at temperature T1 is heated to temperature T2 such that ΔT = T2 − T1 is very small. Let A2 be the surface area of the sheet at temperature T2. Experimentally, it is found that the increase in the
surface area of the sheet (areal expansion), A2 − A1, is proportional to A1 and ΔT. Therefore, (A2 − A1) ∝ A1 ΔT
A2 − A1 = σA1ΔT, where s is the constant of proportionality, called the coefficient of areal expansion of the solid.
σ = (A2 − A1) / (A1 ΔT). It is expressed in per °C.
We have A2 = A1 + σA1ΔT = A1(1 + σΔT).
σ is the increase in the area of a solid per unit original area per unit rise in its temperature.
Volumetric expansion coefficient of a solid :
Suppose a solid with volume V1 at temperature T1 is heated to temperature T2 such that ΔT = T2 − T1 is very small.
Let V2 be the volume of the solid at temperature T2.
Experimentally, it is found that the increase in the volume of the solid (volumetric expansion), V2 - V1, is proportional to V1 and DT.
∴ (V2 − V1) ∝ V1ΔT.
V2 − V1= βV1ΔT, where , b is the constant of proportionality, called the volumetric expansion coefficient of the solid.
β = (V2 − V1) / (V1 ΔT). It is expressed in per °C.
We have V2 = V1 + βV1ΔT = V1 (1 + βΔT).
β is the increase in the volume of a solid per unit original volume per unit rise in its temperature.
[Note : It can be shown that β = (3/2)σ = 3λ]
Expansion of liquids : A liquid does not have a definite shape but it has a definite volume. So we can define a volumetric expansion coefficient for a liquid as follows.
V2 = V1 (1 + βΔT)
Here, ΔT is the change in temperature and V1 and V2 are the initial and final volumes of the liquid. β is the volumetric expansion coefficient of the liquid.
Expansion of gases : A gas does not even have a fixed volume. Gas expands on heating but if the gas is kept in a closed box, its volume cannot increase but its pressure increases.
Therefore, the expansion of a gas is measured by keeping its pressure constant.
This volumetric expansion coefficient is called the constant pressure expansion coefficient and is given by the following formula.
V2 = V1 (1 + βDT)
Here, ΔT is the change in temperature and V1 and V2 are the initial and final volumes of the gas at constant pressure. β is the volumetric expansion coefficient of the gas.
The coefficient of linear expansion of a solid substance,
The coefficient of areal expansion of a solid substance,
The volumetric expansion coefficient of a solid substance,
This formula is also applicable to a liquid. The constant pressure expansion coefficient of a gas (the volumetric expansion coefficient at constant pressure),
When a substance is heated, its volume increases, and if the mass is kept constant by enclosing the substance, the density of the substance decreases as density = mass/volume. |
Useful links :
| Main Page : - Maharashtra Board Class 8th General Science - All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter 13-Chemical Change and Chemical Bond - online Notes Next Chapter : Chapter 15-Sound -online Notes |