Inside the Atom
Based on Maharashtra Board Class 8- General Science - Chapter 5-Notes-Solutions-Videos-Tests-PDF
Notes
Topics to be learn :
- Atomic theory
- Electronic configuration
- Valency and valence electrons
- Nuclear Reactor
Matter : Matter is made up of very small particles. An atom is the smallest unit of matter.
Atom : An atom is the smallest particle of an element which retains its chemical identity in all physical and chemical changes.
Dalton’s atomic theory :
- According to Dalton’s atomic theory matter is made up of atoms and atoms are indivisible and indestructible.
- All atoms of an element are alike while different element have different atom with different mass.
Thomson’s plum pudding model of atom :
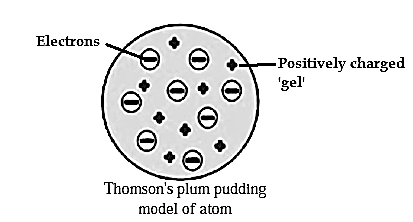
- According to Thomson’s model the positive change is distributed throughout the atom and the negatively charged electron are embedded in it.
- The distributed positive charge is balanced by the negative charge on the electrons. Therefore the atom becomes electrically neutral.
Rutherford’s scattering experiment : Rutherford studied the inside of atom by his celebrated scattering experiment and put forth the nuclear model of atom in the year 1911. Alpha particles emitted by radioactive element bear a positive charge. Rutherford bombarded alpha particles through a very thin gold foil. He observed the path of a - particles by means of a fluorescent screen around the gold foil. It was expected that
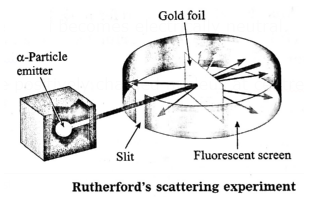
Rutherford’s atomic model : On the basis of the alpha particle experiment the following conclusions were drawn by Rutherford :

- An atom has tiny, dense positively charged nucleus at centre of an atom.
- Most of the mass of the atom is concentrated in the nucleus.
- Negatively charged particles called electrons revolve around the nucleus.
- The total negative charged on all the electron is equal to the positive charge on the nucleus.
- As the opposite charges are balanced the atom is electrically neutral.
- There is an empty space between the revolving electron and the atomic nucleus.
Bohr’s stable orbit atomic model :
- In the year 1913 Danish scientist Niels Bohr explained the stability of atom by putting forth stable orbit atomic model.
- The electrons revolving around the atomic nucleus lie in the concentric circular orbits at certain distance from the nucleus.
- Energy of an electron is constant while it is in a particular orbit.
- When an electron jumps from an inner orbit to an outer orbit it absorbs specific amount of energy, and when it jumps from an outer orbit to an inner orbit it emits specific amount of energy.
- The energy emitted or absorbed during these transitions is equal to energy differences between the initial state and the final state of the electron.
Atomic structure : An atom is formed from the nucleus and the extra nuclear part. These contain three types of subatomic particles.
Atomic number (Z) : The number of electrons or protons in an atom is called the atomic number. It is denoted by Z.
Atomic mass number (A) : The total number of protons and neutrons in the nucleus of the atom is called the atomic mass number. It is denoted by A. Both protons and neutrons are whole numbers, therefore, atomic numbers and atomic mass numbers are always in whole numbers.
Distribution of electron : Electrons revolve in stable shells. These shells have a definite energy. The shell nearest to the nucleus is called the first shell. The next shell is called the second shell. A symbol ‘n’ is used for the ordinal number of a shell. The shells are referred to by the symbols K, L, M, N, corresponding to the ordinal numbers n = 1, 2, 3, 4, The maximum number of electron a shell can contain is obtained by the formula ‘2n2’. As the magnitude of ‘n" increases, the energy of an electron in that shell increases.
Electronic configuration of elements : The electrons in an atom are distributed in the shells according to their maximum capacity. The shell wise distribution of the electron in an atom of an element is called the electronic configuration of that element. Fig-Electronic configuration of atoms
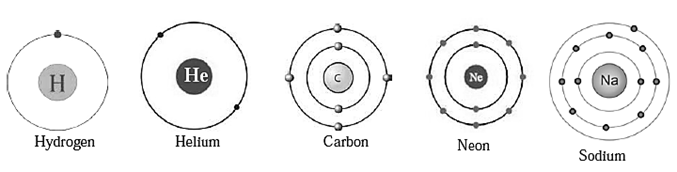
Valency and valence electrons : Valency of an atom is determined by the configuration of its outermost shell. Therefore the outermost shell is called valence shell and electrons in the outermost shell are called valence electrons.
- “Valency of an element is same as the number of its valence electrons if this number is four or less than four.
- On the other hand, when an element has four or more valence electrons, the number of electron by which the octet is short of completion is the valency of that element.
- Atoms of all the elements except inert gases have tendency to combine with other atoms, i.e. they have a non-zero valency.
Isotopes : Atoms of the same element having the same atomic number, but different atomic mass numbers are called isotopes.
Uses of Isotopes :
Nuclear Reactor : Nuclear reactor is a machine that generates electricity on large scale by using atomic energy.
In a nuclear reactor, the nuclear energy in atom is released by bringing about nuclear reactions on the nuclear fuel.
When uranium—235 is bombarded with a slow speed neutron, it undergoes nuclear fission. Various elements are produced. For example : Krypton — 92 and Barium - 141 along with 2 to 3 neutrons are emitted on fission, these neutron have high speed. Their speed is reduced and they are used for bombarding more Uranium—235 nuclei. The process is repeated many times. In this way a chain reaction of nuclear fission takes place (See the figure). A large amount of nuclear energy is released during a chain reaction of fission. The chain reaction is controlled to prevent the probable explosion. Neutrons are slowed down using graphite or heavy water as moderator. The chain reaction is controlled by absorbing neutron with the help of rods of boron, cadmium and beryllium. The heat produced in the fission is taken out by water as coolent. Water is converted into steam. The available heat is used to drive turbines to produce electricity.
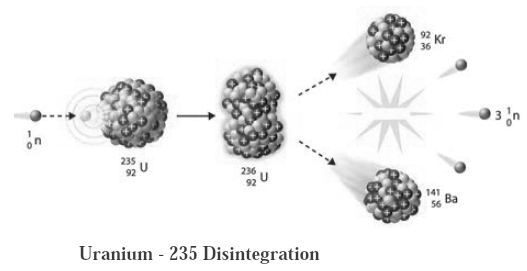
| Moderator : The substance which reduces the speed of fast moving neutrons produced in a fission is called a moderator. Graphite or heavy water is used as moderator for reducing the speed of neutrons.
Controller : To reduce the number of neutron by absorbing them rods of boron, cadmium, beryllium etc. are used as controller. |
Useful links :
| Main Page : - Maharashtra Board Class 8th General Science - All chapters notes, solutions, videos, test, pdf.
Books : MSBSHSE -Class 8th Science Text Books – Chapter wise PDF for download Previous Chapter : Chapter 4: Current Electricity and Magnetism - view online Notes Next Chapter : Chapter 6- Composition of Matter-view online Notes |
Very nice
Very small and important notes
Very nice mam thank you so much 😘😘😘😘😊 mam post the next chapter notes
Very helpful notes , I didn’t get this notes any where but just at this website only I get this notes.Thank you so much for this