Composition of Matter
Maharashtra Board Class 8- General Science - Chapter-6
Solution
Question 1:
Choose the appropriate option and rewrite the following statements.
A. The intermolecular force is _______ in the paricles of solid.
(i) Minimum (ii) Moderate (iii) maximum (iv) indefinite.
B. Solids retain their voume even when external pressure is applied. This property is called__________
(i) plasticity (ii) Incompressibility (iii) fluidity (iv) elasticity
C. Matter is classified into the types mixture, compound and element by applying the criterion______________
(i) states of matter ii Phases of matters iii chemical compositions of matter iv all of these
D. Matter that contain two or more constituent substances is called__________
(i) mixture (ii) compound (iii) element (iv) metalloid
E. Milk is an example of type of matter called __________
(i) solution (ii) homogeneous mixture iii heterogeneous mixture (iv) suspension
F. Water, mercury and bromine are similar o each other, because three are
(i) liquids (ii) compounds (iii) nonmetals (iv) elements.
G. valency of carbon is 4 and that of oxygen is 2. From this, we understand that there are _______ chemical bond/bonds between the carbon atom and one oxygen atom in the compound-carbon dioxide.
(i) 1 (ii) 2 (iii) 3 (iv) 4
A. The intermolecular force is maximumin the paricles of solid. B. Solids retain their voume even when external pressure is applied. This property is called elasticity. C. Matter is classified into the types mixture, compound and element by applying the criterion Chemical composition of matter. D. Matter that contain two or more constituent substances is calledmixture. E. Milk is an example of type of matter called heterogeneous mixture. F. Water, mercury and bromine are similar to each other, because three are liquids. G. Valency of carbon is 4 and that of oxygen is 2. From this, we understand that there are 2 chemical bond/bonds between the carbon atom and one oxygen atom in the compound carbon dioxide.
Question 2:
Identify the odd term out and explain
1. Gold, silver, copper, brass
2. Hydrogen, hydrogen peroxide, carbon dioxide, water vapour.
3. Milk, lemon juice, carbon, steel.
4. water, mercury, bromine, petrol.
5. sugar, slat, baking soda, blue vitrol.
6. Hydrogen, sodium, potassium, carbon.
Question 3:
Answer the following questions.
1. Plants synthesize glucose in sunlight with the help of chlorophyll from carbon dioxide and water and give away oxygen. identify the four compounds in this process and name their types.
Photosynthesis is a chemical process through which plants, some bacteria and algae, produce glucose and oxygen from carbon dioxide and water, using only light as a source of energy, which is absorbed by chlorophyll. 6CO2 + 6H2O → C6H12O6 + 6O2 Four compounds in this process are as follows: 1.Carbon dioxide = Inorganic compound 2.Water = Inorganic compound 3.Glucose = organic compound 4.Chlorophyll = complex compound
2. In one sample of brass, the following ingredients were found : copper (70%) and zinc (30%). Identify the solvent, solute and solution from these.
Brass is an alloy it contains 70% copper, and 30% zinc. Copper is solvent with largest proportion Zinc is solute in smaller proportion The solution is Brass
3. Sea water tastes salty due to the dissolved salt. the salinity (the proportion of salts in water) of some water bodies Lonar lake - 7.9 %, Pacific Ocean 3.5%, Mediterranean sea- 3.8%, Dead sea- 33.7%. Explain two characteristics of mixture from the above information.
Characteristics of mixtures from above information are: 1.Constituent substances of a mixture (proportion of salt in water) do not combine chemically. 2.The proportion of constituent substances present by weight in a mixture can be variable. 3.The constituent of mixture can be separated by physical process.
Question 4:
Give two examples each
A. Liquid element
B. Gaseous element
C. Solid element
D. Homogeneous mixture
E. Colloid
F. Organic compound
G. Complex compound
H. Inorganic compound
I. Metalloid
J. Element with valency 1
K. Element with valency 2
A. Liquid element = mercury (Hg), bromine(Br2) B. Gaseous element = oxygen(O2), nitrogen(N), hydrogen(H) C. Solid element = sodium(Na), carbon (c), aluminium(Al) D.Homogeneous mixture = sugar in water, corn oil, Sea water. E. Colloid = milk, blood, jelly. F. Organic compound = glucose, urea, carbohydrates. G. Complex compound = chlorophyll, hemoglobin, cyanocobalamine. H. Inorganic compound = limestone, rust, common salt, Soda. I. Metalloid = silicon, germanium, arsenic. J .Element with valency 1 = sodium(Na), potassium(K), chlorine(Cl). K. Element with valency 2 = magnesium(Mg), calcium(Ca)
Question 5:
Write the names and symbols of the constituent eleements and identify their valencies from the molecular formulae given below.
KCl, HBr, MgBr2, K2O, NaH, CaCl2, CCl4, HI, H2S, Na2S, FeS, BaCl2
Compounds
Name of compounds
Symbol of constituent elements
Valency of constituent elements
KCl
Potassium chloride
K, Cl
K = 1, Cl = 1
HBr
Hydrogen bromide
K, Br
K = 1, Br = 1
MgBr2
Magnesium bromide
Mg, Br
Mg = 2, Br = 1
K2O
Potassium oxide
K, O
K = 1, O = 2
NaH
Sodium hydride
Na, H
Na = 1, H = 1
CaCl2
Calcium chloride
Ca, Cl
Ca = 2, Cl = 1
CCl4
Carbon tetrachloride
C, Cl
C = 4, Cl = 1
HI
Hydrogen iodide
H, I
H = 1, I = 1
H2S
Hydrogen sulphide
H, S
H = 1, S = 2
Na2S
Sodium sulphide
Na, S
Na = 1, S = 2
FeS
Iron (II) Sulfide
Fe, S
F = 2, S = 2
BaCl2
Barium chloride
Ba, Cl
B = 2, Cl = 1
Question 6:
Chemical composition of some matter is given in the following table. Identify the main type of matter from their.
| Name of matter | Chemical composition | Main type of matter |
| Sea water | H2O + NaCl + MgCl2 | |
| Distilled water | H2O | |
| Hydrogen gas filled in a ballon | H2 | |
| The gas in LPG cylinder | C4H10 + C3H8 | |
| Baking soda | NaHCO3 | |
| Pure gold | Au | |
| The gas in oxygen cylinder | O2 | |
| Bronze | Cu + Sn | |
| Diamond | C | |
| Heated white powder of blue vitroi | CuSO4 | |
| Lime stone | CaCO3 | |
| Dilute hydrochloric acid | HCL+ H2O |
Name of matter
Chemical composition
Main type of matter
Sea water
H2O + NaCl + MgCl2
mixture
Distilled water
H2O
compound
Hydrogen gas filled in a ballon
H2
element
The gas in LPG cylinder
C4H10 + C3H8
mixture
Baking soda
NaHCO3
compound
Pure gold
Au
element
The gas in oxygen cylinder
O2
element
Bronze
Cu + Sn
mixture
Diamond
C
element
Heated white powder of blue vitroi
CuSO4
compound
Lime stone
CaCO3
compound
Dilute hydrochloric acid
HCL+ H2O
mixture
Question 7:
Write scientific reason.
1. Hydrogen is combustible, oxygen helps combustion, but water helps to extinguish fire.
2. Constituent substances of a colloid cannot be separated by ordinary filtration.
Colloidal solution is heterogeneous. Constituent substances of a colloid cannot be separated by ordinary filtration because the size of the particles in a colloids (or colloidal solution) is bigger than those in a true solution but smaller than those in suspension. It is in between 1μ to 100μ in diameter. The pore size of ordinary filter paper is more than 100μ due to which colloidal particles are passed through the pores of a filter paper. Therefore we prefer to use filter paper so that, filtration of colloidal particles take place easily.
3. Lemon sherbat has sweet, sour and salty taste and it can be poured in a glass.
4. A solid matter has the properties of definite shape and volume.
A solid matter has the properties of definite shape and volume because of the following reasons:
Question 8:
Deduce the molecular formulae of the compound obtained from the following pairs of elements by the cross multiplication method.
1. C (Valency 4) & Cl (Valency 1)
Step 1 : Write the symbols of the elements. Step 2 : Write the valency below the respective elements. Step 3 : Cross-multiply symbols of elements with their respective valency. The molecular formula is CCl4
C
Cl
C
Cl
4
1

Step 4 : Write down the chemical formula of the compound.
2. N (Valency 3) & H (Valency 1)
Step 1 : Write the symbols of the elements. Step 2 : Write the valency below the respective elements. Step 3 : Cross-multiply symbols of elements with their respective valency. Step 4 : Write down the chemical formula of the compound. The molecular formula is NH3
N
H
N
H
3
1
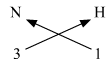
3. C (Valency 4) & O (Valency 2)
Step 1 : Write the symbols of the elements. Step 2 : Write the valency below the respective elements. Step 3 : Cross-multiply symbols of elements with their respective valency. The molecular formula is CO2
C
O
C
O
4
2
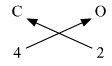
4. Ca (Valency 2) & O (Valency 2)
Step 1 : Write the symbols of the elements. Step 2 : Write the valency below the respective elements. Step 3 : Cross-multiply symbols of elements with their respective valency. Step 4 : Write down the chemical formula of the compound.
Ca
O
Ca
O
2
2
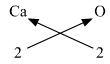
The molecular formula is CaO
Useful links :
| Main Page : - Maharashtra Board Class 8th General Science - All chapters notes, solutions, videos, test, pdf.
Books : MSBSHSE -Class 8th Science Text Books – Chapter wise PDF for download Videos : Maharashtra Board Class 8th General Science Videos - watch chapter wise topic wise videos of all chapters Previous Chapter : Chapter 5: Inside The Atom - view online Solution Next Chapter : Chapter 7- Metals and Nonmetals - Coming Soon |