Metals and Non-Metals
Maharashtra Board Class 8- General Science - Chapter-7
Solution
Question 1:
Complete the table
| Property of metal | Use in everyday life |
| i. Ductility | |
| ii. Malleability | |
| iii. Conduction of heat | |
| iv. Conduction of electricity | |
| v. Sonority |
Property of metal
Use in everyday life
i. Ductility
Gold, Silver Ornaments, Electrical Copper & Aluminium wire.
ii. Malleability
Aluminium sheets, GI Sheets.
iii. Conduction of heat
Cooking wares, Copper vessels, electric press, Boilers
iv. Conduction of electricity
Copper wires, Aluminium wires
v. Sonority
Cymbals, doorbells, Brass articles
Question 2:
Identify the odd term-
1-Gold, silver, iron, diamond.
2-Ductility, brittelness, sonority, malleability.
3-Carbon, bromic, sulphur, phosphorus.
4-Brass, bronze, iron, steel.
1-Diamond is odd one out because Diamond is non-metal and others are Metals. 2-Brittleness is odd one out because it is a property of non-metals and rest are the properties of metals. 3-Bromine is odd one out because it is liquid non-metal and others are solid non-metals. 4-Iron is odd one out because it is not an alloy and others are alloys.
Question 3:
Write scientific reasons.
A. The stainless steel vessels in Kitchen have copper coating on the bottom.
Hence, the stainless steel vessels in kitchen have copper coating on the bottom.
B. Copper and brass vessels are cleaned with lemon.
D. Sodium metal is kept in kerosene.
Sodium metal is kept in kerosene because sodium is very reactive metal. It is kept in kerosene to prevent it from coming in contact with oxygen and moisture.
Question 4:
Answer the following
A. What is done to prevent corrosion of metals?
By applying a layer of paint, oil, grease or varnish on the surface of a metal to prevent corrosion. Also plating with non-corroding metal is done. By galvanizing process, iron is coated with thin layer of zinc. Due to these processing the contact of metal surface with air is lost and corrosion is prevented. By applying a powder coating. In this process, a dry powder is applied to the clean metal surface to avoid its contact with surrounding oxygen.
B. What are the metals that make the alloys brass and bronze ?
The alloy brass is formed from copper and zinc. The alloy bronze is formed from copper and tin.
C. What are the adverse effects of corrosion ?
(1) Damage of metallic equipment (2) A reddish coloured deposit (rust) is formed on iron by reaction with oxygen gas, (3) A greenish coloured deposit (copper carbonate) is formed on copper by reaction - with carbon dioxide. (4) A blackish coloured deposit is formed (silver sulphide) on silver. (5) Release of harmful pollutants from iron corrosion that contaminates the air (6) Corrosion causes damages to car bodies, bridges, iron railings, ships specially those of iron, silver articles and copper vessels.
D. What are use of Noble metals. ?
Uses of noble metals : (1) Gold, Silver and Platinum are used to Prepare ornaments. (2) Silver is used in medicines. (it has Antibacterial property). (3) Salts of silver like silver chloride are used for making photographic films. (4) Silver foils are used for decorating sweets. (3) Gold and Silver are also used to make Metals and few electronic devices. (4) Platinum, Palladium metals are used as a catalyst in the manufacture of sulphuric acid and nitric acid.
Question 5:
Three experiments to study the process of rusting are given below. Observe the three test tubes and answer the following questions.
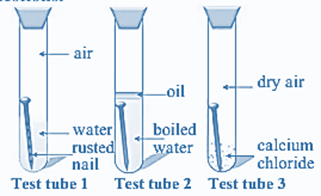
Rusting is caused due to, A. In the test tube 2, oil cuts the supply of air to nail due to which oxidation of nail is prevented and boiled water is free from gases, Hence, the nail in the test tube 2 is not rusted. B.The nail in the test tube 1 is rusted highly because in the test tube 1, iron nail meets with all the requirements which is essential for the process of corrosion. The nail is in contact with water and air. The oxidation process is fast. C. The nail in the test tube 3 is not rusted because calcium chloride is one of the best absorbent. So, it absorbs all the moisture and making air dry. Hence corrosion process is not take place.
Useful links :
| Main Page : - Maharashtra Board Class 8th General Science - All chapters notes, solutions, videos, test, pdf.
Books : MSBSHSE -Class 8th Science Text Books – Chapter wise PDF for download Videos : Maharashtra Board Class 8th General Science Videos - watch chapter wise topic wise videos of all chapters Previous Chapter : Chapter 6: Composition of Matter - view online Solution Next Chapter : Chapter 8- Pollution - online Solution |